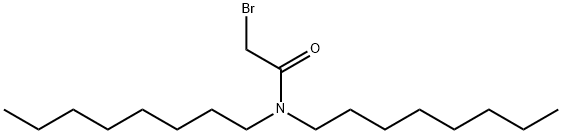
Acetamide, 2-bromo-N,N-dioctyl- synthesis
- Product Name:Acetamide, 2-bromo-N,N-dioctyl-
- CAS Number:145570-18-9
- Molecular formula:C18H36BrNO
- Molecular Weight:362.39

1120-48-5
273 suppliers
$12.00/10g
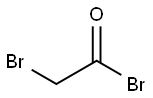
598-21-0
312 suppliers
$10.00/10g

145570-18-9
2 suppliers
inquiry
Yield:145570-18-9 93%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran;
Steps:
3 Synthesis of the cationic lipid pcTG40 of formula I in which R3, R4=ethylene chain; R1, R2=methyl; m=p=1; R5=R6=(C8H17)2NC(=O)
A solution of bromoacetyl bromide (0.69 ml; 7.94 mmol) in tetrahydrofuran (2 ml) at 0° C. is added to a mixture of dioctylamine (2.00 ml; 6.62 mmol) and diisopropylethylamine (1.73 ml; 9.93 mmol) in tetrahydrofuran (13 ml). The mixture is stirred for 1 h at room temperature and is then diluted with ether (25 ml) and washed with aqueous 10% hydrochloric acid solution (10 ml). The organic phase is washed with water, dried over sodium sulfate, concentrated under reduced pressure and purified by chromatography on a column of silica gel (eluent: 85/15 hexane/ether) to give 2-bromo-N,N-dioctylacetamide (2.23 g; 93%) in the form of a brown oil. 1H NMR (200 MHz, CDCl3): δ3.83 (s, 2H, BrCH2-C(O)-); 3.31 and 3.27 (2t, J=6.5 Hz, 4H, -CH2)2N-C(O)-); 1.57 (m, 4H, -CH2-CH2)2N-C(O)-); 1.28 (s, 20H, -CH2-): 0.89 and 0.87 (2t, J=6.4 Hz, 6H, Me-).
References:
US6291423,2001,B1