
Acetamide, N-[1-(3-bromophenyl)-4-piperidinyl]- synthesis
- Product Name:Acetamide, N-[1-(3-bromophenyl)-4-piperidinyl]-
- CAS Number:1416775-95-5
- Molecular formula:C13H17BrN2O
- Molecular Weight:297.19
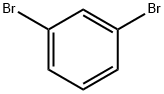
108-36-1
455 suppliers
$10.00/1g
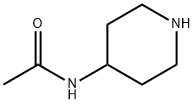
5810-56-0
113 suppliers
$43.60/250mg
![Acetamide, N-[1-(3-bromophenyl)-4-piperidinyl]-](/CAS/20210111/GIF/1416775-95-5.gif)
1416775-95-5
0 suppliers
inquiry
Yield:1416775-95-5 80%
Reaction Conditions:
with sodium t-butanolate;tris-(dibenzylideneacetone)dipalladium(0);2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in toluene at 80; for 17 h;Inert atmosphere;
Steps:
34.1 Synthesis of N-[1-(3-bromophenyl)-4-piperidyl]acetamide
Example 34Synthesis of N-[1-(3-{3-[4-(1-aminocyclobutyl)phenyl]-2-(2-aminopyridin-3-yl)-3H-imidazo[4,5-b]pyridin-5-yl}phenyl)piperidin-4-yl]-N-methylacetamide trihydrochlorideStep 1Synthesis of N-[1-(3-bromophenyl)-4-piperidyl]acetamide A mixture of 1,3-dibromobenzene (7.98 mL, 49.2 mmol), N-(4-piperidyl)acetamide (7.00 g, 49.2 mmol), Pd2(dba)3 (2.25 g, 2.46 mmol), (rac)-BINAP (2.30 g, 3.69 mmol) and sodium tert-butoxide (5.68 g, 59.1 mmol) in toluene (492 mL) was heated at 80° C. for 17 hours under nitrogen. After cooling to room temperature, the mixture was diluted with EtOAc and filtered through a Celite pad. The combined filtrate and washings were concentrated. The residue was purified by silica gel column chromatography (hexane/AcOEt=50:50 to 0:100) to afford the desired product (11.6 g, 80%) as pale orange solid.500 M Hz 1H-NMR (CDCl3) δ: 7.09 (1H, t, J=8.3 Hz), 7.04 (1H, t, J=1.7 Hz), 6.95-6.93 (1H, m), 6.83 (1H, dd, J=8.3, 1.7 Hz), 5.37-5.35 (1H, m), 3.99-3.91 (1H, m), 3.64-3.60 (2H, m), 2.88 (2H, td, J=12.6, 2.3 Hz), 2.06-2.01 (2H, m), 1.99 (3H, s), 1.56-1.48 (2H, m);LCMS: 297, 299 [M+H].
References:
US2012/329791,2012,A1 Location in patent:Page/Page column 74