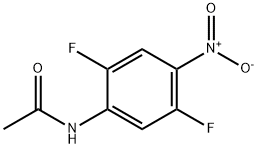
Acetamide, N-(2,5-difluoro-4-nitrophenyl)- synthesis
- Product Name:Acetamide, N-(2,5-difluoro-4-nitrophenyl)-
- CAS Number:366-53-0
- Molecular formula:C8H6F2N2O3
- Molecular Weight:216.14
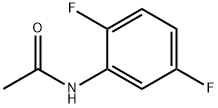
398-90-3
63 suppliers
$75.00/1g

366-53-0
3 suppliers
inquiry
Yield:366-53-0 90.6%
Reaction Conditions:
with nitric acid at 0; for 2 h;
Steps:
3
To a stirred HNCb solution (21.9 g, 333.27 mmol, 15.63 mL, 96% purity, 11.41 equiv) was added dropwise compound 3.2 (5.00 g, 29.22 mmol, 1 equiv) at 0 °C. After addition was complete, the reaction mixture was stirred at 0 °C for 2 hr. LC-MS and TLC (petroleum ether/ethyl acetate = 1/1, Rf = 0.61) showed compound 3.2 was consumed, and the main product was compound 3.3 (r.t. = 0.746 min, [M+H]+ = 216.7). The reaction mixture was poured into ice water (50 mL), stirred for 10 min and then filtered. The resulting cake was washed with water (50 mL x 3) and dried under reduced pressure to give compound 3.3 (5.72 g, 26.46 mmol, 90.6% yield) as a yellow solid. NMR (400 MHz DMSO-de): d 10.47 (br s, 1H), 8.36 (dd, J = 6.5, 14.1 Hz, 1H), 8.21 (dd, J = 7.0, 10.8 Hz, 1H), 2.20 (s, 3H).
References:
WO2020/41270,2020,A1 Location in patent:Paragraph 0352; 0354
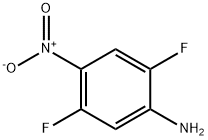
1542-36-5
46 suppliers
$95.00/100mg

366-53-0
3 suppliers
inquiry
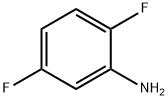
367-30-6
296 suppliers
$11.00/1g

366-53-0
3 suppliers
inquiry