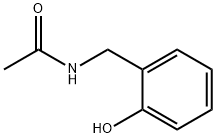
Acetamide, N-[(2-hydroxyphenyl)methyl]- synthesis
- Product Name:Acetamide, N-[(2-hydroxyphenyl)methyl]-
- CAS Number:80311-94-0
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
![AcetaMide, N-[(2-Methoxyphenyl)Methyl]-](/CAS/GIF/63452-53-9.gif)
63452-53-9
![Acetamide, N-[(2-hydroxyphenyl)methyl]-](/CAS/20180601/GIF/80311-94-0.gif)
80311-94-0
N-(2-methoxybenzyl)acetamide (6.5 g, 0.036 mol) was used as starting material and dissolved in dichloromethane (DCM, 100 mL). The solution was cooled to -10 °C under nitrogen protection. Subsequently, boron tribromide (BBr3, 15 mL, 0.15 mol) was slowly added dropwise. After the dropwise addition was completed, the ice bath was removed and the reaction mixture was allowed to react at room temperature with stirring for 2 hours. Upon completion of the reaction, the mixture was again cooled to -10 °C and water (20 mL) was slowly added to quench the reaction. The reaction mixture was extracted with dichloromethane (3 x 100 mL), the organic layers were combined, washed sequentially with water (3 x 100 mL) and brine (100 mL), and dried to give N-(2-hydroxybenzyl)acetamide (4.3 g, 71.7% yield) as a solid product. The product did not require further purification and could be used directly in the subsequent reaction. The product was characterized by 1H NMR (300 MHz, DMSO-d6) and LC-MS: 1H NMR δ 9.56 (brs, 1H), 8.27 (brs, 1H), 7.09-7.03 (m, 2H), 6.79-6.72 (m, 2H), 4.16 (d, J = 6.0 Hz, 2H), 1.87 (s, 3H); LC- MS m/z 166.2 [M + H]+, retention time tR = 1.15 min.
![AcetaMide, N-[(2-Methoxyphenyl)Methyl]-](/CAS/GIF/63452-53-9.gif)
63452-53-9
1 suppliers
inquiry
![Acetamide, N-[(2-hydroxyphenyl)methyl]-](/CAS/20180601/GIF/80311-94-0.gif)
80311-94-0
25 suppliers
$94.00/100mg
Yield:80311-94-0 71.7%
Reaction Conditions:
with boron tribromide in dichloromethane at -10 - 20; for 2 h;Inert atmosphere;
Steps:
Preparation of N-(2-hydroxybenzyl)acetamide
N-(2-methoxybenzyl)acetamide (6.5 g, 0.036 mol) was dissolved in DCM (100 mL), andthe solution was cooled to -lOoC under nitrogen. To this solution was added BBr3 (15 mL, 0.15mol) dropwise. After addition, the ice bath was removed and the reaction mixture was stirred atroom temperature for 2 hours. The reaction mixture was re-cooled to -lOoC and quenched by5 water (20 mL). The mixture was extracted by DCM (3 x 100 mL), and the organic layer waswashed with water (3 x 100 mL), brine (100 mL) and dried to give the title compound (4.3 g,71.7 %) as a solid. It was used directly in the next step without further purification. lH NMR(300 MHz, DMSO): 8 9.56 (brs, lH), 8.27 (brs, lH), 7.09- 7.03 (m, 2H), 6.79- 6.72 (m, 2H),4.16 (d, 2H, J = 6.0 Hz), 1.87 (s, 3H). LC-MS: 166.2 [M+H]+, tR = 1.15 min.
References:
WO2013/110578,2013,A1 Location in patent:Page/Page column 45; 46
6850-57-3
229 suppliers
$10.00/1g

108-24-7
0 suppliers
$14.00/250ML
![Acetamide, N-[(2-hydroxyphenyl)methyl]-](/CAS/20180601/GIF/80311-94-0.gif)
80311-94-0
25 suppliers
$94.00/100mg
![Acetamide, N-[[2-(acetyloxy)phenyl]methyl]-](/CAS/20210305/GIF/91133-29-8.gif)
91133-29-8
0 suppliers
inquiry
![Acetamide, N-[(2-hydroxyphenyl)methyl]-](/CAS/20180601/GIF/80311-94-0.gif)
80311-94-0
25 suppliers
$94.00/100mg
932-30-9
184 suppliers
$10.00/250mg
![Acetamide, N-[(2-hydroxyphenyl)methyl]-](/CAS/20180601/GIF/80311-94-0.gif)
80311-94-0
25 suppliers
$94.00/100mg
![Benzaldehyde, 2-hydroxy-, oxime, [C(E)]- (9CI)](/CAS/20180808/GIF/21013-96-7.gif)
21013-96-7
9 suppliers
inquiry
![Acetamide, N-[(2-hydroxyphenyl)methyl]-](/CAS/20180601/GIF/80311-94-0.gif)
80311-94-0
25 suppliers
$94.00/100mg