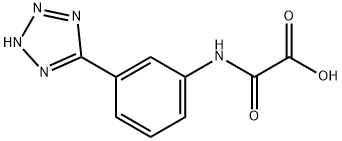
Acitazanolast synthesis
- Product Name:Acitazanolast
- CAS Number:114607-46-4
- Molecular formula:C9H7N5O3
- Molecular Weight:233.18

79-37-8
460 suppliers
$17.67/10gm:
73732-51-1
83 suppliers
$35.35/250mgs:

114607-46-4
62 suppliers
inquiry
Yield: 74.8%
Reaction Conditions:
in 1,2-dimethoxyethane;water;ethyl acetate
Steps:
2 Preparation of 3-(1H-tetrazol-5-yl)Oxanilic Acid
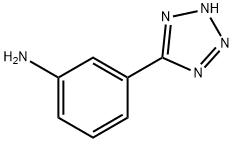
EXAMPLE 2 Preparation of 3-(1H-tetrazol-5-yl)Oxanilic Acid Oxalyl chloride (12 g) was dissolved in 50 ml of anhydrous dimethoxyethane and a solution of 3-(1H-tetrazol-5-yl)aniline (5 g) in 250 ml of anhydrous dimethoxyethane was dropwise added to the solution obtained above over 3 hours at room temperature while stirring. Insolubles were removed by filtering the solution, then 50 ml of water was gradually added to the reaction mixture under ice cooling and stirring was continued for one hour at room temperature. Then, 500 ml of ethyl acetate was added thereto to carry out extraction, the extract was washed with water, dried over anhydrous sodium sulfate and then the solvent was distilled off to obtain 5.4 g of the objective product, 3-(1H-tetrazol-5-yl)oxanilic acid (yield 74.8%). The physical properties and spectral data of this product were consistent with those described in EXAMPLE 1.
References:
Wakamoto Pharmaceutical Co., Ltd. US4795754, 1989, A