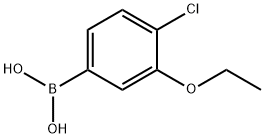
AKOS BRN-1022 synthesis
- Product Name:AKOS BRN-1022
- CAS Number:900174-62-1
- Molecular formula:C8H10BClO3
- Molecular Weight:200.43
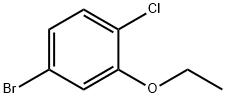
900174-61-0

900174-62-1
The general procedure for the synthesis of 4-chloro-3-ethoxyphenylboronic acid from 4-bromo-1-chloro-2-ethoxybenzene was as follows: n-butyllithium (n-BuLi, 1.6 M hexanes solution, 13.6 mL, 21.8 mmol) was slowly added to a solution of tetrahydrofuran (THF, 20 mL) of Intermediate 15A (3.8 g, 16 mmol) at -78 °C. The reaction mixture was stirred continuously at -78 °C for 40 min, followed by the addition of triisopropyl borate (7.43 mL, 32 mmol). The reaction system was slowly warmed from -78 °C to room temperature and stirring was continued for 18 hours. Upon completion of the reaction, the reaction was quenched with 1.0 N hydrochloric acid (50 mL) followed by extraction of the organic phase with ethyl acetate (EtOAc). The organic phase was washed with saturated saline and dried over anhydrous sodium sulfate (Na2SO4). The crude product was purified by fast column chromatography (eluent ratio of dichloromethane: ethyl acetate: methanol = 50:50:1) to give 1.85 g (57% yield) of Intermediate 15B as a white solid. Its 1H NMR (400 MHz, CDCl3) data were as follows: δ 1.53 (t, J=7.03Hz, 3H), 4.23 (d, J=7.03Hz, 2H), 7.48 (d, J=7.91Hz, 1H), 7.66 (d, J=6.15Hz, 2H).

900174-61-0
35 suppliers
$40.00/1g

900174-62-1
52 suppliers
$45.00/100mg
Yield:900174-62-1 57%
Reaction Conditions:
Stage #1: 1-bromo-4-chloro-3-ethoxybenzenewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.666667 h;
Stage #2: with Triisopropyl borate in tetrahydrofuran;hexane at -78; for 18 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;hexane;
Steps:
15.15B
To a solution of Intermediate 15A (3.8 g, 16 mmol) in THF (20 mL) at -78° C. was added n-BuLi (1.6 M in hexanes, 13.6 mL, 21.8 mmol). The mixture was stirred at -78° C. for 40 min before triisopropyl borate (7.43 mL, 32 mmol) was added. The reaction was left stirring from -78° C. to rt over 18 h. It was quenched with 1.0 N HCl (50 mL), extracted with EtOAc, washed with brine and dried over Na2SO4. The crude residue was purified by flash column chromatography (CH2Cl2: EtOAc:MeOH=50:50:1) to give 1.85 g (57%) of Intermediate 15B as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm 1.53 (t, J=7.03 Hz, 3 H) 4.23 (d, J=7.03 Hz, 2 H) 7.48 (d, J=7.91 Hz, 1 H) 7.66 (d, J=6.15 Hz, 2 H).
References:
US2007/3539,2007,A1 Location in patent:Page/Page column 43-44