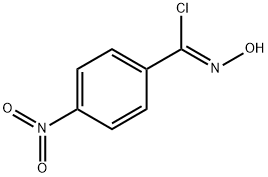
ALPHA-CHLORO-4-NITROBENZALDOXIME synthesis
- Product Name:ALPHA-CHLORO-4-NITROBENZALDOXIME
- CAS Number:58402-81-6
- Molecular formula:C7H5ClN2O3
- Molecular Weight:200.58
1129-37-9
89 suppliers
$18.00/1g

58402-81-6
4 suppliers
inquiry
Yield:58402-81-6 90%
Reaction Conditions:
with N-chloro-succinimide in N,N-dimethyl-formamide at 0 - 20; for 48 h;Inert atmosphere;
Steps:
(Z)-O-Methyl-4-nitrobenzohydroximoyl chloride (42).
General procedure: Compound 42 was prepared by a procedure similar to that of Sanders.18 To a solution of (E)-4-nitrobenzaldehyde O-methyl oxime (41) (0.90 g, 4.59 mmol) in dimethylformamide (25 ml) at 0 °C was added portionwise Nchlorosuccinimide (6.13 g, 4.59 mmol), and the mixture stirred at room temperature for 48 h. The solution was diluted with water (100 ml), extracted with ethyl acetate (3 × 100 ml), the combined organic phases washed with water (3 × 100 ml), dried over anhydrous sodium sulfate, filtered and the solvent removed in vacuo. Purification by flash chromatography (hexane:ethyl acetate 49:1) afforded 42 exclusively as the (Z) isomer (white solid; 0.39 g, 1.80 mmol, 39%).
References:
Rennison, David;Conole, Daniel;Tingle, Malcolm D.;Yang, Junpeng;Eason, Charles T.;Brimble, Margaret A. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 24,p. 6629 - 6635] Location in patent:supporting information

1129-37-9
89 suppliers
$18.00/1g

58402-81-6
4 suppliers
inquiry
555-16-8
525 suppliers
$5.00/5g

58402-81-6
4 suppliers
inquiry