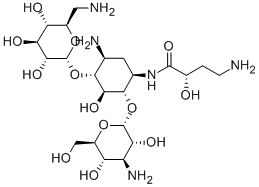
AMIKACIN synthesis
- Product Name:AMIKACIN
- CAS Number:37517-28-5
- Molecular formula:C22H43N5O13
- Molecular Weight:585.6
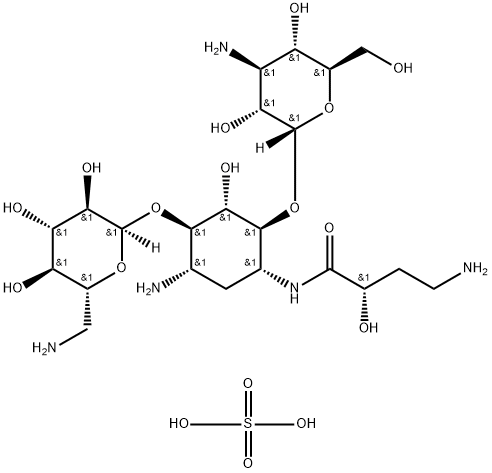
39831-55-5
485 suppliers
$5.00/1g

37517-28-5
260 suppliers
$43.00/1g
Yield:-
Reaction Conditions:
with Amberlite ion exchange resin (OH-form) in water; for 1 h;
Steps:
1
Example 1Preparation of l-7V-[4-(tert-butoxycarbonyl)amino-2-S-hydroxybutyryl]kanamycinAmikacin sulfate 1.0 grams (Commercially available from a number of chemical companies: O. M. Chemical Co., Ltd., Shanghai, China; Zhejiang Winsun Imp. & Exp. Co., Ltd, Zhejiang, China; Ningbo DHY Pharmaceutical Co., Zhejiang, China; and Shanghai Xudong Haipu Pharmaceutical Co., Ltd, Shanghai, China) was treated with 4 grams of Amberlite ion exchange resin (OH- form, Aldrich, Milwaukee, Wi) in 10 mL of water for 1 hour. The resultant solution was filtered and lyophilized to give Amikacin Free Base. The Amikacin free base (5.0 g, 8.5 mmoles) was dissolved in a mixture of 1,4-dioxane (165 mL) and H2O (235 mL). To this solution was added tert- butyloxycarbonyloxy-5-norbornene-2,3-dicarboxamide (1.3 g, 7.8 mmoles) dissolved in a mixture of CH2Cl2 (10 mL) and 1,4-dioxane (5 mL), over 50 min at room temperature. The reaction was stirred at room temp for 3 hours, and then evaporated under reduced pressure. The resultant solid was subjected to silica gel chromatography (4:4:2, MeOH, CH2Cl2, NH4OH, Rf = 0.25) and then lyophilized to give the the title compound, 1.6 g (30%) as a white solid. 1H NMR (D2O, 300 MHz) δ 5.45 (d, IH, J = 3.3 Hz), 5.05 (d, IH, J =3.3 Hz), 4.06 (m, IH), 3.94 (m, 2H), 3.7 (m, 4H), 3.6-3.5 (m, 5H), 3.4-3.0 (m, 7H), 1.99 (m, IH), 1.87 (m, IH), 1.66 (m, IH), 1.52 (m, IH), 1.34 (s, 9H). 13C NMR (D2O, 75 MHz) δ 175.3 (HABA, C=O), 157.2 (Boc, C=O), 97.0, 96.3, 81.2, 80.1 (Boc, quaternary), 79.5, 73.1, 71.3, 71.0, 70.2, 70.0, 68.3, 68.0, 67.7, 65.6, 58.9 (CH2), 53.9, 48.2, 47.3, 39.4 (CH2), 35.5 (CH2), 32.44 (CH2), 31.7 (CH2), 26.7. LCMS Method A; (retention time, m/z) 2.14 min, 686.2 (M+H) and 708.1 (M+Na). Starting material (Rf = 0.10) was also recovered (1.7 g, 38%). In addition, one more compound was recovered (Rf = 0.74) and had a mass spectrum consistent with 6'-N-(fert-butoxycarbonyl)-l-Λ/-[4- (ter^butoxycarbonyl)amino-2-£-hydroxybutyryl]-kanamycin A (1.47 g, 24%). LCMS Method A; (retention time, m/z) 2.68 min, 820.2 (M+H) and 842.2 (M+Na).
References:
WO2010/30690,2010,A1 Location in patent:Page/Page column 17-18