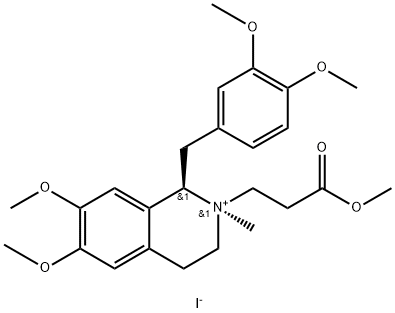
Atracurium EP Impurity D synthesis
- Product Name:Atracurium EP Impurity D
- CAS Number:1075726-91-8
- Molecular formula:C25H34INO6
- Molecular Weight:571.45
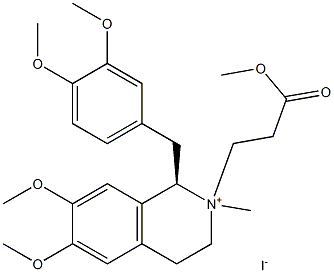
1075726-86-1
19 suppliers
inquiry

1075726-91-8
10 suppliers
inquiry
Yield:1075726-91-8 32%
Reaction Conditions:
in methanol;ethyl acetate at 5 - 25; for 73 h;Purification / work up;Heating / reflux;
Steps:
9B
EXAMPLE 9B[0120] This example describes the preparation of (lR-cis)-l-[(3,4-dimethoxy- phenyl)methyl]-l,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-2-methoxycarbonylethyl- isoquinolinium iodide by crystallization of isomeric mixture. [0121] A mixture of the cis and the trans isomers of (lR)-l-[(3,4-dimethoxy- phenyl)methyl]-l,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-2-methoxycarbonylethyl- isoquinolinium iodide (1.0 g), prepared as described in example 9, was admixed with methanol (4 ml) and heated to obtain a solution. Ethyl acetate (6 ml) was added and the solution was cooled to about 25°C and stirred for 1 hour. Subsequently, the mixture was cooled to 5°C and kept at that temperature for 72 hours. The thus formed crystals were collected by filtration, washed with ethyl acetate and dried to afford (lR-cis)-l-[(3,4- dimethoxyphenyl)methyl]-l,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-2-methoxycarbonyl-
References:
WO2008/132746,2008,A1 Location in patent:Page/Page column 28-29