AVE-8134 synthesis
- Product Name:AVE-8134
- CAS Number:304025-09-0
- Molecular formula:C22H23NO5
- Molecular Weight:381.42
Yield:304025-09-0 50%
Reaction Conditions:
with potassium tert-butylate in toluene at -20; for 6.16667 h;
Steps:
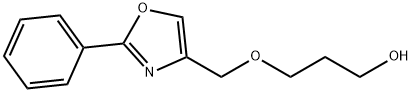
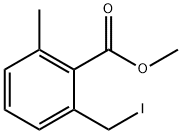
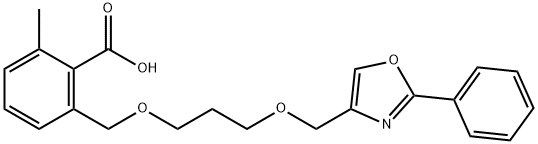
5 EXAMPLE 5; 2-Methyl-6-[3-(2-phenyloxazol-4-ylmethoxy)propoxymethyl]benzoic acid
4.8 g of methyl 2-chloromethylmethylbenzoate are dissolved at room temperature in 250 ml of acetone and admixed with 35 g of sodium iodide. The mixture is heated to reflux for 6 h. Subsequently, the solvent is removed at 0° C. in vacuo. The residue is analyzed by means of LC-MS (87.7 area % of methyl 2-iodomethyl-6-methylbenzoate) and dissolved in 20 ml of toluene. The solution is added dropwise at -20° C. within 10 min to a mixture of 5.0 g of 3-(2-phenyloxazol-4-ylmethoxy)propan-1-ol, 4.8 g of potassium tert-butoxide and 30 ml of toluene. Afterwards, the mixture is stirred at -20° C. for 6 h and diluted with 100 ml of water, and the aqueous phase is removed. The organic phase is admixed with 40 ml of NMP and 10 ml of 32% sodium hydroxide solution and heated to reflux for 8 h on a water separator. Subsequently, the mixture is admixed with 100 ml of water and extracted twice with 25 ml of M ether each time. The aqueous phase is acidified with 5 ml of acetic acid and extracted twice with 50 ml of ethyl acetate each time. After the phases have been separated, the organic phase is dried over magnesium sulfate and the solvent is removed in vacuo. After the crystallization from diisopropyl ether, 4.2 g of 2-methyl-6-[3-(2-phenyloxazol-4-ylmethoxy)propoxymethyl]benzoic acid (50% of theory, 99.2 HPLC area %) are obtained.
References:
US2004/192956,2004,A1 Location in patent:Page 4