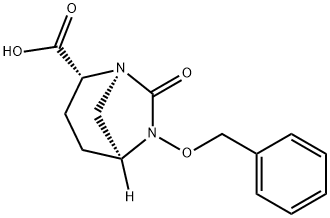
Avibactam Impurity 28 synthesis
- Product Name:Avibactam Impurity 28
- CAS Number:1383814-70-7
- Molecular formula:C14H16N2O4
- Molecular Weight:276.29
![1,6-Diazabicyclo[3.2.1]octane-2-carboxylic acid, 7-oxo-6-(phenylmethoxy)-, compd. with cyclohexanamine (1:1), (1S,2R,5S)-](/CAS/20211123/GIF/1383814-71-8.gif)
1383814-71-8
0 suppliers
inquiry

1383814-70-7
20 suppliers
inquiry
Yield:1383814-70-7 91.5%
Reaction Conditions:
with sodium dihydrogenphosphate in water;
Steps:
24.4
Step 4: (2R,5S)-6-(Benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxylic acid (q) Cyclohexylamine salt 750 mg of (2R,5S)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxylic acid was dissolved in saturated aqueous sodium dihydrogen phosphate solution, followed by extraction three times with ethyl acetate, and the combined organic layer was washed with saturated brine and subsequently dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to afford 507 mg of the title compound as a colorless oil (yield 91.5%). Enantiomeric excess: 98.6% ee. (CHIRALPAK AD-H, 4.6×150 mm, trifluoroacetic acid/hexane/ethanol=0.1/80/20, UV 210 nm, flow rate 1 mL/min., retention time 6.2 min.).[α]20D -11.1° (c 0.90, CHCl3); 1H NMR and MS were equivalent to those of the title compound of Example 9.
References:
US2012/165533,2012,A1 Location in patent:Page/Page column 32
![1,6-Diazabicyclo[3.2.1]octane-2-carboxylic acid, 7-oxo-6-(phenylmethoxy)-, 1,1-dimethylethyl ester, (1S,2R,5S)-](/CAS/20210305/GIF/1383814-69-4.gif)
1383814-69-4
0 suppliers
inquiry

1383814-70-7
20 suppliers
inquiry