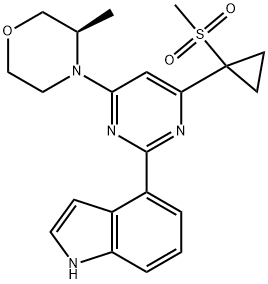
AZ20 synthesis
- Product Name:AZ20
- CAS Number:1233339-22-4
- Molecular formula:C21H24N4O3S
- Molecular Weight:412.51
![Morpholine, 4-[2-chloro-6-[1-(methylsulfonyl)cyclopropyl]-4-pyrimidinyl]-3-methyl-, (3R)-](/CAS/20210305/GIF/1233339-68-8.gif)
1233339-68-8
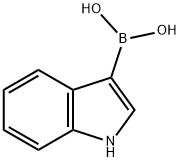
220465-43-0

1233339-22-4
The general steps for the synthesis of (R)-4-(2-(1H-indol-4-yl)-6-(1-(methylsulfonyl)cyclopropyl)pyrimidin-4-yl)-3-methylmorpholine from the compound (CAS:1233339-68-8) and 4-indolylboronic acid were as follows: 1. suspend bis(triphenylphosphine)palladium chloride (1.692 g, 2.41 mmol), (R)-4-(2-chloro-6-(1-(methylsulfonyl)cyclopropyl)pyrimidin-4-yl)-3-methylmorpholine (8.00 g, 24.11 mmol), 1H-indol-4-ylboronic acid (4.27 g 26.52 mmol) and 2M aqueous sodium carbonate (36.2 mL, 72.33 mmol) were heated to 90 °C for overnight reaction. 2. After the reaction was completed, the DME was removed and the reaction mixture was diluted with EtOAc (100 mL). 3. The mixture was washed with water (2 x 100 mL), the organic phase was separated, filtered through a diatomaceous earth pad and concentrated in vacuum to give the crude product. 4. Purify the crude product by silica gel column chromatography with an elution gradient of 0 to 10% EtOAc in DCM solution. The grades containing the target product were combined and the solvent was evaporated. 5. The residue was purified again by silica gel column chromatography with an elution gradient of 0 to 25% EtOAc in DCM solution. The fractions containing the target product were combined and the solvent was evaporated. 6. The residue is dissolved and purified by reversed-phase C18 silica gel column (415g HP C18 column) using a mixture of water (containing 1% NH3) and MeCN as the eluent with decreasing polarity. The grades containing the target product were combined and the solvent was evaporated. 7. The residue was dissolved in anhydrous MeOH, dried over MgSO4, filtered and the solvent evaporated to give a gel. 8. The colloid was dissolved in DCM (500 mL), filtered and the solvent was removed under reduced pressure. 9. The residue was dissolved in MeOH (50 mL), stirred overnight at room temperature, and the resulting precipitate was collected by filtration to afford the target product (R)-4-(2-(1H-indol-4-yl)-6-(1-(methylsulfonyl)cyclopropyl)pyrimidin-4-yl)-3-methylmorpholine (5.10 g, 51% yield). Product characterization: 1H NMR (400 MHz, DMSO-d6) δ 1.29 (3H, d), 1.57-1.64 (2H, m), 1.68-1.78 (2H, m), 3.24-3.31 (1H, td), 3.29 (3H, s), 3.51 (1H, td), 3.67 (1H, dd), 3.80 (1H, d). d), 3.93-4.06 (1H, dd), 4.21 (1H, d), 4.61 (1H, bs), 6.85 (1H, s), 7.21 (1H, t), 7.32 (1H, t), 7.46 (1H, t), 7.56 (1H, d), 8.06 (1H, dd), 11.25 (1H, s); m/z (ESI+ ) MH+, 413.12. Chiral HPLC analysis (HPI 100 system on a 4,5 μm Chiralpak AS-H (250 mm × 4.6 mm) column, elution conditions: isohexane/EtOH/TEA 60/40/0.1) showed an Rf value of 8.815 and a purity of >99%.
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

220465-43-0
167 suppliers
$34.00/1 g

1233339-22-4
121 suppliers
$25.00/1mg
Yield: 51%
Reaction Conditions:
with sodium carbonate;bis-triphenylphosphine-palladium(II) chloride in 1,2-dimethoxyethane;water at 90;
Steps:
3.01
Example 3.014-{4-r(3R)-3-Methylmorpholin-4-yl1-6-ri-(methylsulfonyl)cvclopropyl1pyrimidin-2-yl}- lH-indoleBis(triphenylphosphine)palladium chloride (1.692 g, 2.41 mmol), (R)-4-(2-chloro-6-(l- (methylsulfonyl)cyclopropyl)pyrimidin-4-yl)-3-methylmorpholine (8.00 g, 24.11 mmol), IH- indol-4-ylboronic acid (4.27 g, 26.52 mmol) and 2M aqueous sodium carbonate (36.2 rnL, 72.33 mmol) were suspended in DME:water 4:1 (170 mL) and heated to 90 0C overnight. The DME was removed and the reaction mixture diluted with EtOAc (100 mL). The mixture was washed with water (2 x 100 mL), the organics separated, filtered through a pad of Celite and concentrated in vacuo on to silica. The residue was purified by chromatography on silica with an elution gradient of 0 to 10% EtOAc in DCM. Fractions containing product were combined and evaporated. The residue was purified by chromatography on silica eluting with a gradient of 0-25% EtOAc in DCM. Fractions containing product were combined and evaporated onto reverse phase Cl 8 silica. The crude product was purified by reverse phase using a 415g HP Cl 8 column using decreasingly polar mixtures of water (containing 1% NH3) and MeCN as eluents. Fractions containing product were combined and evaporated. The residue was taken up in dry MeOH and dried over MgSO4. The mixture was filtered and the solvent evaporated, leaving a gum. The gum was dissolved in DCM (500 mL), filtered and the solvent removed under reduced pressure. The residue was dissolved in MeOH (50 mL) and allowed to stir at RT overnight. The resultant precipitate was collected by filtration to afford the title compound (5.1O g, 51%); 1H NMR (400 MHz, DMSO-/): 1.29 (3H, d), 1.57 - 1.64 (2H, m), 1.68 - 1.78 (2H, m), 3.24-3.31 (IH, td), 3.29 (3H, s), 3.51 (IH, td), 3.67 (IH, dd), 3.80 (IH, d), 3.93 - 4.06 (IH, dd), 4.21 (IH, d), 4.61 (IH, bs), 6.85 (IH, s), 7.21 (IH, t), 7.32 (IH, t), 7.46 (IH, t), 7.56 (IH, d), 8.06 (IH, dd), 11.25 (IH, s); m/z: (ESI+) MH+, 413.12. Chiral HPLC: (HPI lOO System 4, 5μm Chiralpak AS-H (250mm x 4.6mm) column eluting with iso- Hexane/EtOH/TEA 60/40/0.1) Rf, 8.815 >99%.
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED;FOOTE, Kevin, Michael;NISSINK, Johannes, Wilhelmus, Maria WO2010/73034, 2010, A1 Location in patent:Page/Page column 68-69
![Morpholine, 4-[2-chloro-6-[(methylsulfonyl)methyl]-4-pyrimidinyl]-3-methyl-, (3R)-](/CAS/20210305/GIF/1233339-73-5.gif)
1233339-73-5
0 suppliers
inquiry

1233339-22-4
121 suppliers
$25.00/1mg
1233339-71-3
4 suppliers
inquiry

1233339-22-4
121 suppliers
$25.00/1mg
6299-85-0
189 suppliers
$10.00/1g

1233339-22-4
121 suppliers
$25.00/1mg
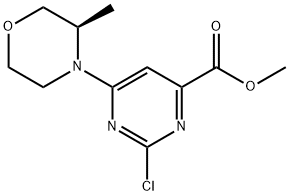
1233339-69-9
8 suppliers
inquiry

1233339-22-4
121 suppliers
$25.00/1mg