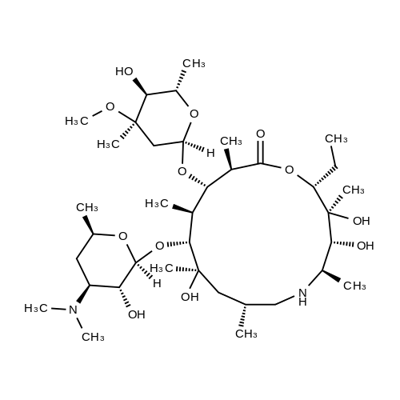
Azathramycin synthesis
- Product Name:Azathramycin
- CAS Number:76801-85-9
- Molecular formula:C37H70N2O12
- Molecular Weight:734.96
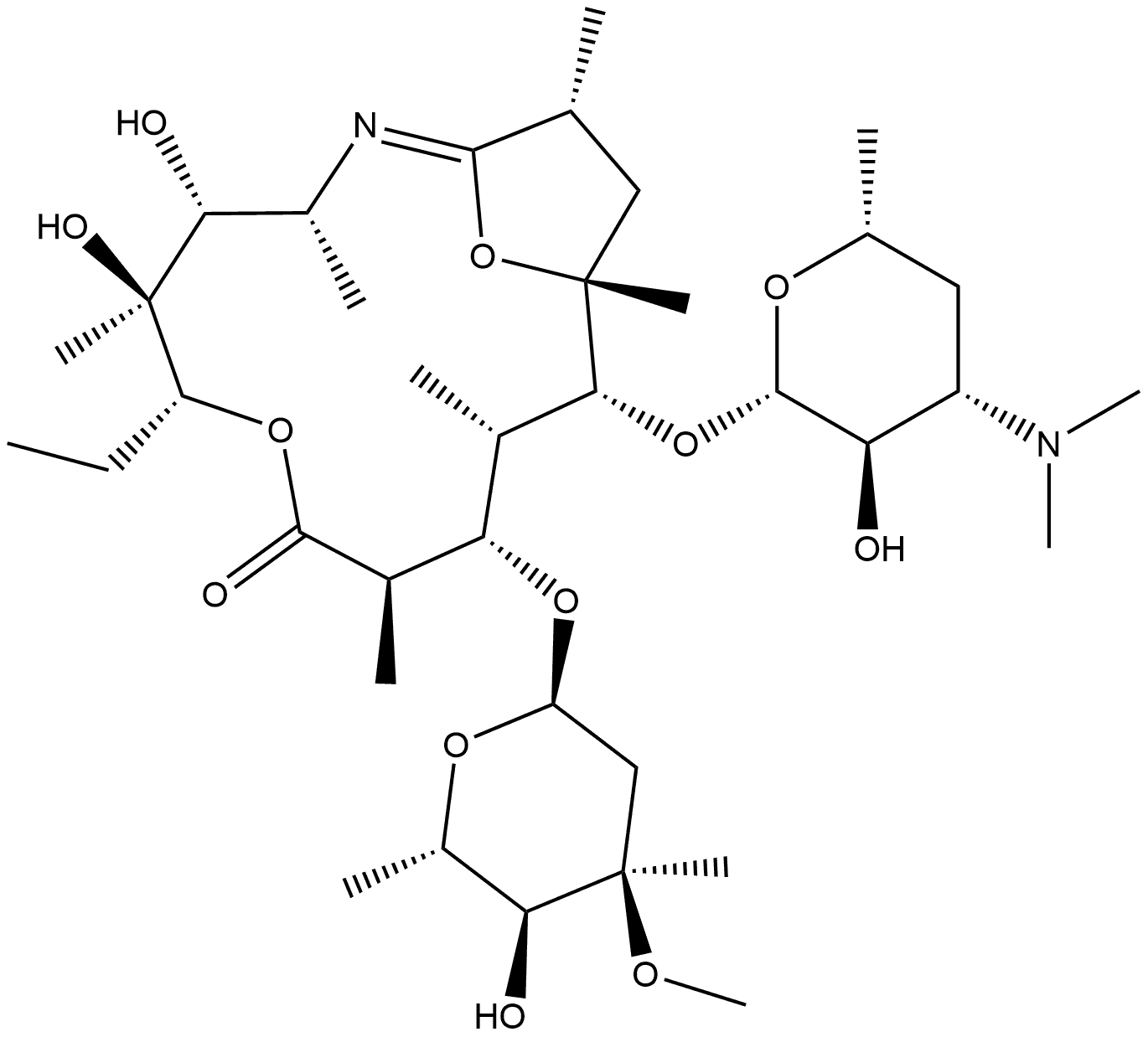
342371-84-0

76801-85-9
Example-2; [93] [94] Preparation of 9-deoxy-9a-azo-9a-homoerythromycin A; Acetic acid was added dropwise to a methanolic (700 mL) suspension of 6,9-imino ether (II) (70.0 g), and the pH was adjusted to 5-6. Subsequently, an aqueous solution of PtO2 (7.0 g) was added to the above mixture and the reaction was carried out under a hydrogen pressure of 8-9 kg/cm2 and a temperature of 40-45°C for 4-5 hours for the hydrogenation reduction reaction. After completion of the reaction, the reaction mixture was filtered through Hyflo Super Cel and the filter cake was washed with methanol. The filtrates were combined and the methanol was removed by distillation under reduced pressure. To the residue, deionized water (700 mL) was added and the pH was adjusted to 11-12 with aqueous sodium hydroxide.The precipitated solid was filtered, drained, and dried in an oven to afford 9-deoxy-9a-azo-9a- homoerythromycin A(III) (55.0 g) in 85% yield.

342371-84-0
1 suppliers
inquiry

76801-85-9
255 suppliers
$5.00/5mg
Yield:76801-85-9 93%
Reaction Conditions:
with potassium borohydride in methanol at 0 - 5; for 2 h;
Steps:
1; 2 Example 1
Add a erythromycin A 6,9-imino ether (40g, 55mmoL) to a 500mL three-necked flask, add 200mL methanol and stir to dissolve, cool to 0 ° C, and add (8.9g, 165mmol) KBH4 and (1.99g, 5.5mmoL), control temperature is not higher than 5 ,The reaction was stirred for 2h. 200 mL of water was added to the reaction mixture, and the solution was adjusted to pH = 3 with 10% hydrochloric acid.After stirring for 30 minutes, add 100 mL of isobutyl acetate and 45 g of IRA-743 resin, and then adjust the pH = 9.5 with 20% sodium hydroxide solution. After stirring for 15 minutes, collect the resin by filtration.The filtrate was left to separate and separated, and the organic layer was separated.It was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain 38.2 g of a white foamy solid with a yield of 93%.
References:
Qingdao Agricultural University;Li Yuwen;Ma Cuili;Liu Yandi CN110684056, 2020, A Location in patent:Paragraph 0006-0007
1357466-70-6
3 suppliers
inquiry

76801-85-9
255 suppliers
$5.00/5mg
111321-02-9
102 suppliers
$25.00/1mg

76801-85-9
255 suppliers
$5.00/5mg
13127-18-9
198 suppliers
$49.00/100mg

76801-85-9
255 suppliers
$5.00/5mg
![Erythromycin, 9-[O-[(4-methylphenyl)sulfonyl]oxime], (9E)- (9CI)](/CAS/20211123/GIF/227948-37-0.gif)
227948-37-0
1 suppliers
inquiry

76801-85-9
255 suppliers
$5.00/5mg