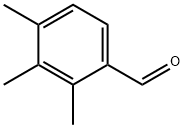
Benzaldehyde, 2,3,4-trimethyl- (6CI,8CI,9CI) synthesis
- Product Name:Benzaldehyde, 2,3,4-trimethyl- (6CI,8CI,9CI)
- CAS Number:34341-28-1
- Molecular formula:C10H12O
- Molecular Weight:148.2
13061-96-6
304 suppliers
$6.00/1g
4885-02-3
194 suppliers
$10.00/1g
526-75-0
216 suppliers
$10.00/1g

34341-28-1
12 suppliers
inquiry
Yield:34341-28-1 97%
Reaction Conditions:
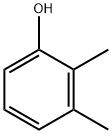
Stage #1: 2,3-Dimethylphenolwith aluminum (III) chloride in dichloromethane; for 0.166667 h;Inert atmosphere;
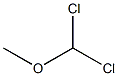
Stage #2: Dichloromethyl methyl ether in dichloromethane at 20; for 0.166667 h;Inert atmosphere;
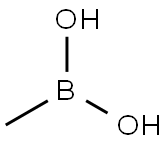
Stage #3: dihydroxy-methyl-boraneFurther stages;
Steps:
General Procedure for Formylation and General Procedure for Trifluoromethanesulfonate Synthesis and General Procedure for Suzuki Cross-Coupling
General procedure: Aluminium trichloride (4.82 g, 36 mmol) was added to asolution of phenol (33 mmol) in anhydrous CH2Cl2 (50 mL)under argon, and the solution was stirred for 10 min.Dichloromethyl methyl ether (3.3 mL, 36 mmol) was addeddropwise via a syringe pump (7.7 mL/h). The reaction wasleft to stir for a further 10 min before cold H2O (200 mL) wasadded slowly. After stirring for a further 10 min, the organiclayer was separated and washed with brine (100 mL) andH2O (150 mL), dried over MgSO4, filtered, and concentratedto give the aldehyde which was purified by columnchromatography. Triethylamine (4.04 g, 40 mmol) was added to a solution ofphenol (13 mmol) in anhydrous CH2Cl2 (13 mL) at -78 °Cunder argon, and the solution stirred for 30 min.Trifluoromethanesulfonic anhydride (2.5 mL, 15 mmol, 1.1equiv) was added dropwise via a syringe pump (7.7 mL/h).The reaction was left to stir for a further 2 h. The reactionmixture was diluted with CH2Cl2 (15 mL) and washed withsat. NaHCO3 (20 mL), brine (20 mL), and H2O (20 mL),dried (MgSO4), filtered, and concentrated to give thetrifluoromethanesulfonate which was purified by columnchromatography. K2CO3 (397 mg, 3 mmol) and PdCl2(dppf)·CH2Cl2 (116 mg,10 mol%) were added to a solution of aryl trifluoromethanesulfonate(1 mmol) in THF (25 mL) which was left to stir for5 min. H2O (HPLC grade, 1.25 mL) was added, followed bymethylboronic acid (255 mg, 4 mmol). The reaction washeated at reflux overnight. EtOAc (7 mL) was added, and the organic layer was separated and washed with H2O (2 × 10mL), dried over MgSO4, filtered, and concentrated to givethe aldehyde which was purified by columnchromatography.
References:
Dhankher, Persis;Sheppard, Tom D. [Synlett,2014,vol. 25,# 3,art. no. ST-2013-D0950-L,p. 381 - 384] Location in patent:supporting information
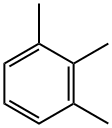
526-73-8
163 suppliers
$32.00/5mL

34341-28-1
12 suppliers
inquiry
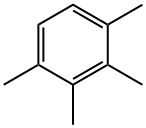
488-23-3
152 suppliers
$13.00/1g

34341-28-1
12 suppliers
inquiry
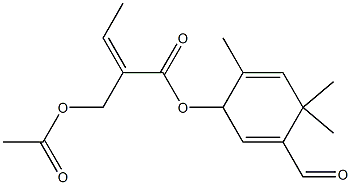
24047-77-6
0 suppliers
inquiry

34341-28-1
12 suppliers
inquiry