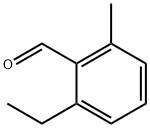
Benzaldehyde, 2-ethyl-6-methyl- (9CI) synthesis
- Product Name:Benzaldehyde, 2-ethyl-6-methyl- (9CI)
- CAS Number:106976-44-7
- Molecular formula:C10H12O
- Molecular Weight:148.2
![1-Butanamine, N-[(2-chloro-6-methylphenyl)methylene]-, [N(E)]-](/CAS/20211123/GIF/945408-08-2.gif)
945408-08-2
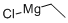
2386-64-3

106976-44-7
c) The synthesis of 2-ethyl-6-methylbenzaldehyde was carried out with reference to the method described in Synthesis 1999, 2138-2144. At 0 °C, 3.20 g (15.3 mmol) of butyl-[1-(2-chloro-6-methylphenyl)-methyl-(E)-ylidene]-amine was dissolved in 30 mL of tetrahydrofuran and 0.19 g (1.53 mmol) of manganese(II) chloride was added. Subsequently, 15.3 mL (30.5 mmol) of an ether solution of 2M ethylmagnesium chloride was added slowly and dropwise while maintaining the temperature of the reaction mixture at 5-10°C. The reaction was completed with stirring. After the dropwise addition was completed, the reaction mixture was continued to be stirred for 90 minutes, during which the temperature was raised to 50 °C (exothermic reaction). Upon completion of the reaction, the reaction was quenched by dropwise addition of water and subsequently diluted with ethyl acetate. The mixture was washed sequentially with water and saturated brine, the organic phase was separated, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. The residue was purified by fast chromatography (silica gel, ethyl acetate/heptane gradient) to give 1.88 g (83% yield) of 2-ethyl-6-methylbenzaldehyde as a yellow oil.1H-NMR (CDCl3): δ 1.26 (3H, t, CH3), 2.60 (3H, s, CH3), 2.98 (2H, q, CH2), 7.09 (1H, d , ArH), 7.12 (1H, d, ArH), 7.35 (1H, dd, ArH), 10.6 (1H, s, CHO).
Yield:106976-44-7 83%
Reaction Conditions:
manganese(ll) chloride in tetrahydrofuran;diethyl ether at 0 - 50; for 1.5 h;
Steps:
57.c
c) 2-Ethyl-6-methyl-benzaldehvdeThis compound was prepared using methodology described in Synthesis 1999, 2138-2144. To a solution of 3.20 g (15.3 mmol) butyl-[l-(2-chloro-6-methyl-phenyl)-meth-(E)- ylidene] -amine in 30 ml tetrahydrofuran at 0 0C was added 0.19 g (1.53 mmol) manganese(II) chloride. 15.3 ml (30.5 mmol) of a 2 M solution of ethylmagnesium chloride in diethyl ether was then added dropwise while the temperature of the reaction mixture was maintained at 5-10 0C. After the addition was complete, the reaction mixture was stirred for a further 90 minutes, during which time the temperature rose to 50 0C (exotherm). The reaction mixture was then quenched by dropwise addition of water before being diluted with ethyl acetate. The mixture was then washed sequentially with water and with saturated brine. The phases were separated and the organic phase was dried over Na2SO4, filtered and concentrated in vacuo. The residue was purified by flash chromatography (silica gel, ethyl acetate/heptane gradient) to afford 1.88 g (83%) of the title compound as a yellow oil. 1H-NMR (CDCl3): 1.26 (3H, t, CH3), 2.60 (3H, s, CH3), 2.98 (2H, q, CH2), 7.09 (IH, d, ArH), 7.12 (IH, d, ArH), 7.35 (IH, dd, ArH), 10.6 (IH, s, CHO).
References:
WO2007/85556,2007,A2 Location in patent:Page/Page column 50
106976-43-6
8 suppliers
inquiry

106976-44-7
16 suppliers
inquiry
65232-55-5
22 suppliers
$51.00/250mg

106976-44-7
16 suppliers
inquiry

106976-50-5
10 suppliers
inquiry

106976-44-7
16 suppliers
inquiry

106987-84-2
2 suppliers
inquiry

106976-44-7
16 suppliers
inquiry