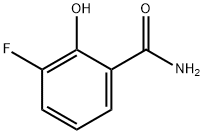
Benzamide, 3-fluoro-2-hydroxy- (9CI) synthesis
- Product Name:Benzamide, 3-fluoro-2-hydroxy- (9CI)
- CAS Number:705949-54-8
- Molecular formula:C7H6FNO2
- Molecular Weight:155.13
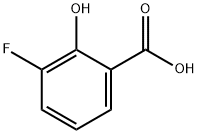
341-27-5
152 suppliers
$10.00/1g

705949-54-8
23 suppliers
inquiry
Yield:705949-54-8 216 mg
Reaction Conditions:
with ammonium hydroxide;1,1'-carbonyldiimidazole in N,N-dimethyl-formamide at 20; for 0.5 h;
Steps:
152.a (a) 3-Fluoro-2-hydroxybenzamide
A 20 mL reaction vial equipped with a stirbar and needle-vented septum cap was charged with 3-fluoro-2-hydroxybenzoic acid (0.568 g) and 1 ,1 '-carbonyldiimidazole (1 .180 g). The solid materials were taken up in Ν,Ν-dimethylformamide (DMF) (2.65 mL) and treated with cone, aqueous ammonia (2.62 mL). After the foaming had subsided, the resulting mixture was stirred 30 minutes at room temperature. The mixture was then transferred to a 100 mL conical flask and diluted with water to approximately 30 mL total volume and then treated with glacial acetic acid until the pH was approximately 6-7. The aqueous mixture/suspension was then extracted with ethyl acetate twice, the organics pooled, dried over sodium sulfate, filtered, and concentrated to a residue. The residue was then triturated with dichloromethane to afford the titled compound as a brilliant yellow solid (216 mg). LCMS m/z 156.0 (M+H)+. NMR (400 MHz, DMSO-c/6) δ ppm 6.85 (td, J=8.08, 4.80 Hz, 1 H) 7.39 (ddd, J=1 1 .12, 8.08, 1 .52 Hz, 1 H) 7.68 (dt, J=8.21 , 1 .33 Hz, 1 H) 8.14 (br. s., 1 H) 8.56 (br. s., 1 H) 13.47 (br. s., 1 H).
References:
WO2017/153952,2017,A1 Location in patent:Page/Page column 213
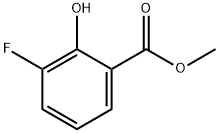
70163-98-3
69 suppliers
$28.00/250mg

705949-54-8
23 suppliers
inquiry
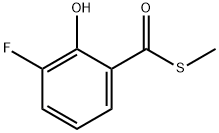
854914-89-9
0 suppliers
inquiry

705949-54-8
23 suppliers
inquiry
367-12-4
433 suppliers
$5.00/10g

705949-54-8
23 suppliers
inquiry

148872-79-1
14 suppliers
inquiry

705949-54-8
23 suppliers
inquiry