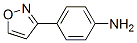
Benzenamine, 4-(3-isoxazolyl)- (9CI) synthesis
- Product Name:Benzenamine, 4-(3-isoxazolyl)- (9CI)
- CAS Number:765912-47-8
- Molecular formula:C9H8N2O
- Molecular Weight:160.1726
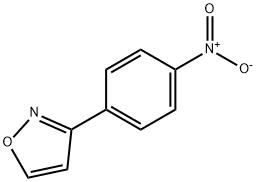
4264-05-5
25 suppliers
$88.00/200mg
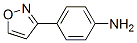
765912-47-8
6 suppliers
inquiry
Yield:765912-47-8 93%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20; for 14 h;
Steps:
3 EXAMPLE 3 - PREPARATION OF 4-(ISOXAZOL-3-YL)ANILINE (3)
To a solution of 3-(4-nitrophenyl)isoxazole (500 mg, 2.63 mmol) in MeOH (30 mL) was added 10% Pd/C (70 mg, 10% wt) under nitrogen atmosphere at room temperature. The reaction mixture was stirred under a hydrogen atmosphere at room temperature for 14 h. Then, the reaction mixture was filtered, and the filtrate was concentrated under vacuum to afford the title compound 3 as a brown sticky oil (550 mg, 93%).XH NMR (400 MHz, DMSO- d6) δ 8.83 (d, J= 1.35 Hz, 1H), 7.54 (d, J= 8.53 Hz, 2H), 6.92 (d, J= 1.35 Hz, 1H), 6.63 (d, J = 8.53 Hz, 2H), 5.53 (s, 2H).
References:
WO2016/73895,2016,A1 Location in patent:Paragraph 00221