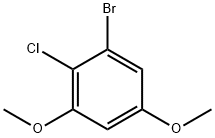
Benzene, 1-bromo-2-chloro-3,5-dimethoxy- synthesis
- Product Name:Benzene, 1-bromo-2-chloro-3,5-dimethoxy-
- CAS Number:81574-72-3
- Molecular formula:C8H8BrClO2
- Molecular Weight:251.5
20469-65-2
254 suppliers
$5.00/1g

81574-72-3
5 suppliers
inquiry
Yield:81574-72-3 75%
Reaction Conditions:
with N-chloro-succinimide;chloro-trimethyl-silane in acetonitrile at 20; for 1 h;regioselective reaction;
Steps:
General procedure for the electrophilic aromatic halogenation. 4-bromo-2-chloro-1-methoxybenzene (2a-Cl) and 2,4-dichloro-1-methoxybenzene (2a-diCl)
General procedure: To a solution of 4-bromoanisole (200.8 mg, 1.09 mmol, 1.0 equiv) in acetonitrile (2 mL) was added N-chlorosuccinimide(NCS) (158.3 mg, 1.19 mmol, 1.1 equiv) at rt to give a slightly cloudy mixture. Chlorotrimethylsilane (TMSCl) (14 μL, 0.11 mmol, 0.1 equiv) was then added drop-wise to the reaction mixture. Within a few minutes, the reaction mixture became clear pale yellow solution. The mixture continued to stir at rt for 1 h and was diluted with hexane. The biphasic mixture was concentrated on a rotary evaporator to a crude white solid-oil mixture. This mixture was taken up in hexane and filtered through a short plug of SiO2 and eluted with 5-10% EtOAc-hexane solution. The clear filtrate was concentrated to obtain a mixture of 4-bromo-2-chloro-1-methoxybenzene (2a-Cl) and 2,4-dichloro-1-methoxybenzene (2a-diCl) 237.0 mg (88% of 2a-Cl and 11% of 2a-diCl,based on NMR ratio 2a-Cl: 2a-diCl = 7.1: 1.0; as a pale yellow solid).
References:
Maibunkaew, Tapanee;Thongsornkleeb, Charnsak;Tummatorn, Jumreang;Bunrit, Anon;Ruchirawat, Somsak [Synlett,2014,vol. 25,# 12,art. no. ST-2014-U0267-L,p. 1769 - 1775] Location in patent:supporting information