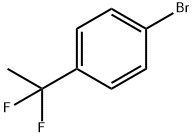
Benzene, 1-bromo-4-(1,1-difluoroethyl)- synthesis
- Product Name:Benzene, 1-bromo-4-(1,1-difluoroethyl)-
- CAS Number:1000994-95-5
- Molecular formula:C8H7BrF2
- Molecular Weight:221.04
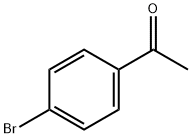
99-90-1

1000994-95-5
Example 63A Synthesis of 1-bromo-4-(1,1-difluoroethyl)benzene: Under argon protection, 3.0 g (15.07 mmol) of 1-(4-bromophenyl)ethanone was dissolved in 30 mL of dichloromethane, followed by the slow addition of 15.9 mL (120.57 mmol) of [ethyl (trifluoro-lambda4-thioalkyl)amino]ethane (DAST). The reaction mixture was slowly warmed to 50 °C and stirred at this temperature overnight. Upon completion of the reaction, the reaction mixture was slowly poured into ice water. The organic phase was separated and the aqueous phase was extracted three times with dichloromethane. The organic phases were combined and dried with magnesium sulfate. After filtration, the solvent was removed by concentration under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/dichloromethane=4:1) to afford 2.56 g (11.58 mmol, 77% yield) of 1-bromo-4-(1,1-difluoroethyl)benzene as a pale yellow liquid. gC-MS (Method 1): rt = 2.84 min; m/z = 220/222 (M)+.

99-90-1
476 suppliers
$6.00/25g

1000994-95-5
100 suppliers
$32.00/250mg
Yield:1000994-95-5 77%
Reaction Conditions:
with diethylamino-sulfur trifluoride in dichloromethane at 50;Inert atmosphere;
Steps:
63A
Example 63A1-Bromo-4-(1,1-difluoroethyl)benzene; Under argon, 3.0 g (15.07 mmol) of 1-(4-bromophenyl)ethanone were initially charged in 30 ml of dichloromethane, and 15.9 ml (120.57 mmol) of [ethyl(trifluoro-λ4-sulphanyl)amino]ethane (DAST) were added slowly. The reaction solution was then slowly warmed to 50° C. and stirred at this temperature overnight. After the reaction had ended, the reaction solution was slowly poured into ice-water. The organic phase was then separated off, and the aqueous phase was extracted three more times with dichloromethane. The combined organic phases were dried over magnesium sulphate. After filtration, the solvent was removed under reduced pressure. The crude product was purified chromatographically on silica gel (mobile phase petroleum ether/dichloromethane 4:1). This gave 2.56 g (11.58 mmol, 77% of theory) of the title compound as a yellowish liquid.GC-MS (Method 1): Rt=2.84 min; m/z=220/222 (M)+.
References:
US2012/172448,2012,A1 Location in patent:Page/Page column 34
139139-81-4
38 suppliers
$121.00/1g
115134-88-8
0 suppliers
inquiry

1000994-95-5
100 suppliers
$32.00/250mg