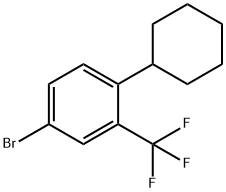
Benzene, 4-bromo-1-cyclohexyl-2-(trifluoromethyl)- synthesis
- Product Name:Benzene, 4-bromo-1-cyclohexyl-2-(trifluoromethyl)-
- CAS Number:1449291-53-5
- Molecular formula:C13H14BrF3
- Molecular Weight:307.15
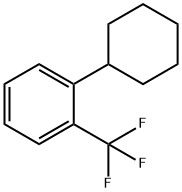
13376-27-7
9 suppliers
inquiry

1449291-53-5
3 suppliers
inquiry
Yield:1449291-53-5 49.6 g
Reaction Conditions:
with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;sulfuric acid in acetic acid at 0 - 5;
Steps:
3 Synthesis of 4-Bromo-1-cyclohexyl-2-trifluoromethylbenzene
1-Cyclohexyl-2-trifluoromethyl-benzene (38.8g ) was dissolved in 126.4 trifluoroacetic acid at 20-25°C. Then the solution was cooled to IT = 0-5°C and 17.19g H2S04 (ca 96%) were added. To the resulting orange suspension 26.73g 1 ,3-dibromo-5,5-dimethylhydantoin were added in 6 portions within 1 -2h at IT = 0-5°C. Thirty minutes after the last addition, an in process control was performed and more 1 ,3-dibromo-5,5-dimethylhydantoin was added on an as needed basis. When the bromination was complete, 67.5g heptanes were added and the mixture was stirred for 5-10 minutes (min.) followed by phase separation at IT = 20 - 25°C. The lower inorganic phase was extracted a second time with 33.8ml heptanes. The combined organic phases were extracted with 57.85g 10% Na-hydrogensulfite in water followed by 55.4g 2N NaOH and three times 41 g water. Charcoal (0.61 g ) was added to the organic phase and the mixture was stirred 1 h at RT. After filtration, the filtrate was dried by isotropic distillation and evaporated to dryness. This gave 49.6g of 4-bromo-1-cyclohexyl-2-trifluoromethyl-benzene as a yellow oil, which was used without further purification for the synthesis of 4-cyclohexyl-3-trifluoromethyl-benzoic acid. 1H-NMR (400MHz, DMSO-d6): δ 1.2-1 .9(1 OH, m), 2.76(1 H,T), 7.55-7.61 (1 H,d), 7.76.7.85(2H,m)
References:
WO2013/113915,2013,A1 Location in patent:Page/Page column 27
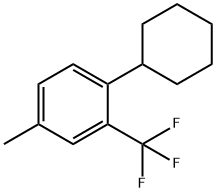
1449293-48-4
1 suppliers
inquiry

1449291-53-5
3 suppliers
inquiry
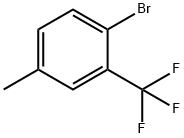
261952-20-9
114 suppliers
$27.00/1g

1449291-53-5
3 suppliers
inquiry
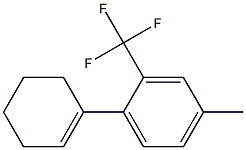
1449293-47-3
0 suppliers
inquiry

1449291-53-5
3 suppliers
inquiry
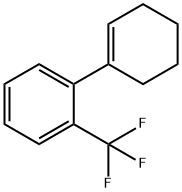
13376-26-6
4 suppliers
inquiry

1449291-53-5
3 suppliers
inquiry