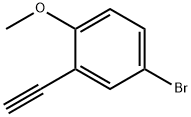
Benzene, 4-broMo-2-ethynyl-1-Methoxy- synthesis
- Product Name:Benzene, 4-broMo-2-ethynyl-1-Methoxy-
- CAS Number:1057669-94-9
- Molecular formula:C9H7BrO
- Molecular Weight:211.06
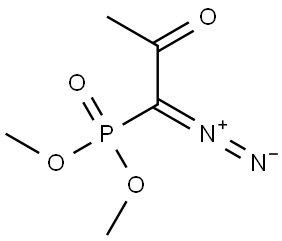
90965-06-3
208 suppliers
$30.25/1g
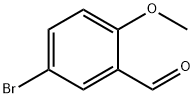
25016-01-7
233 suppliers
$6.00/5g

1057669-94-9
11 suppliers
inquiry
Yield:1057669-94-9 88%
Reaction Conditions:
with potassium carbonate in methanol at 0 - 20; for 6 h;
Steps:
4-Bromo-2-ethynyl-1-methoxybenzene (2)
5-Bromo-ortho-anisaldehyde 1 (2.0 g, 9.3 mmol) and K2CO3 (1.5 g,11 mmol) were dissolved in methanol (40 mL). The mixture was cooled to 0 °C, and the Bestmann-Ohira reagent at 0 °C was added slowly. The mixture was warmed to ambient temperature and stirred for 6 h. After the reaction was completed, the solution was quenched with water, and the solvent was removed in vacuo. The residue was diluted with EtOAc (100 mL), washed with water and brine, and dried over anhydrous MgSO4. The solvent was removed in vacuo, and the residue was purified by column chromatography on deactivated silica gel, with hexane/EtOAc (20:1) as eluent, to obtain the corresponding product 2 (1.72 g, 88 %) as an ivory solid. 1H NMR (600 MHz, CDCl3) δ 3.33 (s, 1H), 3.88 (s, 3H), 6.76 (d, J = 9.0 Hz, 1H), 7.41 (dd, J = 9.0, 2.4 Hz, 1H), 7.56 (d, J = 2.4 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ 56.3, 78.7, 82.5, 112.3, 112.4, 113.4, 133.2, 136.5, 159.9.
References:
Sun, Wei;Kim, Taek-Soo;Choi, Nam Song;Seo, Seung-Yong [Bulletin of the Korean Chemical Society,2018,vol. 39,# 1,p. 126 - 129]
98273-59-7
85 suppliers
$29.00/250mg

1057669-94-9
11 suppliers
inquiry