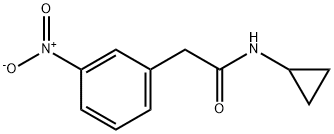
Benzeneacetamide, N-cyclopropyl-3-nitro- synthesis
- Product Name:Benzeneacetamide, N-cyclopropyl-3-nitro-
- CAS Number:1456714-23-0
- Molecular formula:C11H12N2O3
- Molecular Weight:220.22
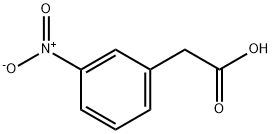
1877-73-2
201 suppliers
$24.00/1g
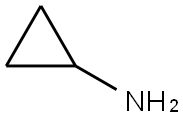
765-30-0
507 suppliers
$10.00/25g

1456714-23-0
2 suppliers
inquiry
Yield:1456714-23-0 2.41 g
Reaction Conditions:
with O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20; for 16 h;Inert atmosphere;
Steps:
ii.a Step a: Synthesis of N-cyclopropyl-2-(3-nitrophenyl)acetamide
To a stirred solution of 2-(3-nitrophenyl)acetic acid (2.5g, 13.80 mmol) in DMF (20 ml) was added HBTU (4.19 g, 16.56 mmol), Ν,Ν-diisopropyl ethylamine (4.82 ml, 27.6 mmol) followed by addition of cyclopropylamine (1.946 ml, 27.6 mmol) at room temperature under nitrogen atmosphere. Reaction mixture was stirred at room temperature for 16 hrs, the reaction mixture was diluted with water (80.0 mL) and extracted with ethyl acetate (20.0 ml x 3). Combined organic layers were washed with cold water (20.0 ml) and brine (10.0 ml); dried over sodium sulphate. The solvent was evaporated under vacuum to give the crude compound which was purified by column chromatography to afford titled compound (2.41 gm). iH NMR (400 MHz, DMSO-de) 8 8.25 (d, 1H, J=2.4Hz), 8.12-8.09 (m, 1H), 7.67 (d, 1H, J=7.6Hz), 7.60 (t, 1H, J=7.6Hz), 3.52 (s, 2H), 2.63-2.58(m, 1H), 0.63-0.55 (m, 2H), 0.41-0.37(m, 2H),. GCMS: 221.09 [M+]
References:
WO2013/136249,2013,A1 Location in patent:Page/Page column 44-45