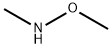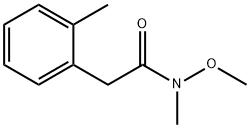
Benzeneacetamide, N-methoxy-N,2-dimethyl- synthesis
- Product Name:Benzeneacetamide, N-methoxy-N,2-dimethyl-
- CAS Number:918417-27-3
- Molecular formula:C11H15NO2
- Molecular Weight:193.24
Yield:918417-27-3 96%
Reaction Conditions:
Stage #1: o-methylphenylacetic acid;O,N-dimethyl-hydroxylamine hydrochloride in chloroform; for 0.166667 h;Cooling with ice;
Stage #2: with N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride in chloroform; for 0.166667 h;
Stage #3: with triethylamine in chloroform; for 3.16667 h;
Steps:
36.a
Example 36 Production of N-benzyl-2-[3-isobutyl-5-methyl-3,4-dihydroisoquinolin-2(1H)-yl]ethanamine a) Production of 2-(2-toluyl)-N-methoxy-N-methylacetamide; [Show Image] A solution of 2.60 g of 2-methylphenyl acetic acid and 2.53 g of N,O-dimethylhydroxylamine monohydrochloride in 50 mL of chloroform was stirred under ice-cooling for 10 minutes, and 4.98 g of WSC monohydrochloride was added thereto, followed by stirring for 10 minutes. 5.25 g of triethylamine was further added thereto, followed by stirring for 10 minutes, and then reacting at room temperature for 3 hours. After completion of the reaction, the reaction liquid was diluted by addition of 1 N hydrochloric acid under ice-cooling, followed by extraction with chloroform. The chloroform layer was washed with a saturated aqueous sodium bicarbonate solution and then with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue obtained was purified by silica gel column chromatography (hexane:ethyl acetate=2:1) to obtain 3.20 g (yield 96%) of a title compound as a colorless oily substance. 1H-NMR (CDCl3) δ: 2.31 (3H, s), 3.21 (3H, s), 3.61 (3H, s), 3.77 (2H, s), 7.12-7. 21 (4H, m).
References:
EP2143714,2010,A1 Location in patent:Page/Page column 80

6638-79-5
594 suppliers
$6.00/25g
10166-09-3
54 suppliers
$45.00/50mg

918417-27-3
4 suppliers
inquiry
644-36-0
333 suppliers
$6.00/5g

918417-27-3
4 suppliers
inquiry