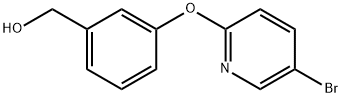
Benzenemethanol, 3-[(5-bromo-2-pyridinyl)oxy]- synthesis
- Product Name:Benzenemethanol, 3-[(5-bromo-2-pyridinyl)oxy]-
- CAS Number:119349-41-6
- Molecular formula:C12H10BrNO2
- Molecular Weight:280.12
Yield:119349-41-6 79%
Reaction Conditions:
with caesium carbonate in dimethyl sulfoxide at 100; for 16 h;
Steps:
33.1
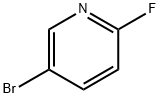
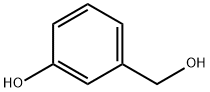
Example 33; Synthesis of 4-{3-[(5-bromopyridin-2-yl)oxy]benzylidene}-N-pyridin-3-ylpiperidine-1-carboxamide; Step 1; 3-(5-Bromopyridin-2-yloxy)phenyl)methanol; 3-Hydroxymethyl-phenol (3.205 g, 25.82 mmol), 5-bromo-2-fluoropyridine (5.00 g, 28.4 mmol) and cesium carbonate (9.26 g, 28.4 mmol) were suspended in DMSO (40 mL) and heated to 100° C. After stirring for 16 h, the reaction mixture was partitioned between water (400 mL) and ethyl acetate (400 mL). The organic layer was separated and the aqueous layer was extracted again with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered and concentrated. The residue was purified by flash chromatography on silica (10-60%, EtOAc:heptane) to afford the desired product (5.71 g, 79% yield) as a clear oil. MS (APCI) M+1=280.0; 1H NMR (400 MHz, CDCl3) δ ppm 1.82 (s, 1H) 4.70 (s, 2H) 6.84 (dd, J=8.58, 0.58 Hz, 1H) 7.00-7.06 (m, 1H) 7.13 (t, J=1.75 Hz, 1H) 7.17-7.23 (m, 1H) 7.38 (t, J=7.80 Hz, 1H) 7.76 (dd, J=8.77, 2.53 Hz, 1H) 8.20 (dd, J=2.63, 0.49 Hz, 1H).
References:
US2008/261941,2008,A1 Location in patent:Page/Page column 27