
Benzenesulfonic acid, 2,3,5,6-tetrafluoro- synthesis
- Product Name:Benzenesulfonic acid, 2,3,5,6-tetrafluoro-
- CAS Number:40707-55-9
- Molecular formula:C6H2F4O3S
- Molecular Weight:230.14
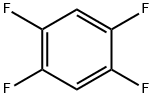
327-54-8
245 suppliers
$10.00/10g

40707-55-9
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with chlorosulfonic acid at 120; for 3 h;Inert atmosphere;
Steps:
63 Example 63 Synthesis of N-cyclopropyl-2,3,5,6-tetrafluoro-N-(4- fluorobenzyl)benzenesulfonamide
A microwave vial was charged with 1,2,4,5-tetrafluorobenzene (6.66 mmol) and chlorosulfonic acid (30 mmol). The vessel was capped and flushed with nitrogen. The reaction mixture was then stirred at 120 °C for 3 hours. The reaction was quenched with ice-cold 1M HCl, and extracted three times with EtOAc. Combined organic fractions were washed with a saturated solution of NaCl, dried over MgSO4 and concentrated in vacuo.2,3,5,6-tetrafluorobenzenesulfonic acid was isolated as a brown oil and used directly in the next step.1H NMR (400 MHz, CDCl3) δ 7.57- 7.49 (tt, J = 9.0, 7.0 Hz, 1H)
References:
WO2019/56120,2019,A1 Location in patent:Paragraph 00134; 00236