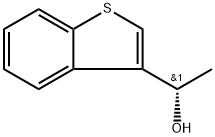
Benzo[b]thiophene-3-methanol, α-methyl-, (αS)- synthesis
- Product Name:Benzo[b]thiophene-3-methanol, α-methyl-, (αS)-
- CAS Number:597553-11-2
- Molecular formula:C10H10OS
- Molecular Weight:178.25
![3-Acetyl benz[b]thiophene](/CAS/GIF/1128-05-8.gif)
1128-05-8
73 suppliers
$45.00/50mg
![Benzo[b]thiophene-3-methanol, α-methyl-, (αS)-](/CAS/20211123/GIF/597553-11-2.gif)
597553-11-2
4 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 3-acetylbenzothiophenewith sodium isopropylate;asymmetric rhodium catalyst in isopropyl alcohol at 20; under 27.7528 - 49.505 Torr; for 2 h;
Stage #2: with acetic acid in isopropyl alcohol;
Steps:
12.2
(2) To a solution of 4.45 g of 1-(1-benzothiene-3-yl)ethanone in 95 mL of isopropanol was added the asymmetric rhodium catalyst solution prepared as described above. Under reduced pressure (about 6600 Pa), an isopropanol solution of sodium isopropoxide (0.1 M, 10.0 mL) was added thereto. Further, the pressure was reduced to about 3700 Pa, followed by stirring at room temperature for 2 hours. To the reaction mixture was added acetic acid (2 mL) to stop the reaction, followed by concentrating under reduced pressure, and the residue was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain (1S)-1-(1-benzothiene-3-yl)ethanol (4.48 g, 94%ee) as a pale yellow oily substance. (HPLC analysis condition: CHIRALCEL OD-H column manufactured by (DAICEL CHEMICAL INDUSTRIES. Ltd., eluding solvent hexane/isopropanol=90/10, flow rate 1.0 mLmin-1, retention time: 8.1 min (S-isomer), 12.6 min (R-isomer)) EI: 178
References:
EP2085383,2009,A1 Location in patent:Page/Page column 23