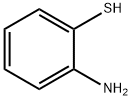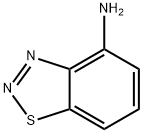
benzo[d][1,2,3]thiadiazol-4-aMine synthesis
- Product Name:benzo[d][1,2,3]thiadiazol-4-aMine
- CAS Number:13599-80-9
- Molecular formula:C6H5N3S
- Molecular Weight:151.19
273-77-8
53 suppliers
$93.00/100mg
![benzo[d][1,2,3]thiadiazol-5-aMine](/CAS/20150408/GIF/1753-29-3.gif)
1753-29-3
14 suppliers
inquiry
![benzo[d][1,2,3]thiadiazol-7-aMine](/CAS/20150408/GIF/CB92729560.gif)
1753-30-6
8 suppliers
inquiry
![benzo[d][1,2,3]thiadiazol-4-aMine](/CAS/20150408/GIF/13599-80-9.gif)
13599-80-9
9 suppliers
inquiry
Yield:1753-30-6 34% ,1753-29-3 10% ,13599-80-9 16%
Reaction Conditions:
Stage #1: benzo[1,2,3]thiadiazolewith sulfuric acid;potassium nitrate at 10 - 20; for 16.5 h;
Stage #2: with hydrogenchloride;iron in ethanol;water; for 1 h;Reflux;
Steps:
B; C
Step C: 7-amino-1 ,2,3-benzothiadiazole. 5-amino-1 ,2,3-benzothiadiazole. and 4-amino-1 .2.3- benzothiadiazoleTo a mixture of nitro-benzo[1 ,2,3]thiadiazoles (35.0 g, 193 mmol) and iron powder (54.1 g, 967 mmol) in ethanol (300 mL) was added concentrated hydrochloric acid(30 mL) and the mixture was heated to reflux for 1 h. The mixture was cooled to RT and the solid was filtered off through Celite. The filtrate was evaporated to a residue which was basified to pH -8 with aqueous sodium carbonate solution, then extracted with 9:1 DCM:methanol (4 x 100 mL). The combined organic fractions were washed with brine (100 mL), dried over sodium sulfate, and concentrated. Purification by column chromatography (10% EtOAc:hexanes) afforded 4.6 g (16%) of 4-amino-1 ,2,3-benzothiadiazole, 10.0 g (34%) of 7-amino-1 ,2,3-benzothiadiazole, and 3.0 g (10%) of 5-amino-1 ,2,3-benzothiadiazole.4-amino-1 ,2,3-benzothiadiazole: 1H NMR (300 MHz, CDCI3): 5.09 (br. s, 2H), 6.74 (d, J=7.5 Hz, 1 H), 7.31 (d, 8.1 Hz, 1 H), 7.42 (t, J=7.5 Hz, 1 H)7-amino-1 ,2,3-benzothiadiazole: 1 H NMR (300 MHz, CDCI3): 4.10 (br. s, 2H), 6.90 (dd, J=7.5, 0.6 Hz, 1 H), 7.46 (t, J=7.5 Hz, 1 H), 8.08 (dd, J=7.5 Hz, 0.6 Hz, 1 H).5-amino-1 ,2,3-benzothiadiazole: 1H NMR (300 MHz, CDCI3): 4.06 (brs, 2H), 7.09 (dd, J=9.0 Hz, 2.5 Hz, 1 H), 7.78 - 7.84 (m, 2H).
References:
WO2011/100502,2011,A1 Location in patent:Page/Page column 49

13599-79-6
0 suppliers
inquiry
![benzo[d][1,2,3]thiadiazol-4-aMine](/CAS/20150408/GIF/13599-80-9.gif)
13599-80-9
9 suppliers
inquiry