
Benzofuran-5-carboxylic acid synthesis
- Product Name:Benzofuran-5-carboxylic acid
- CAS Number:90721-27-0
- Molecular formula:C9H6O3
- Molecular Weight:162.14
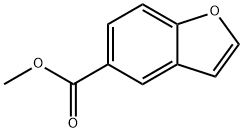
108763-47-9

90721-27-0
General procedure for the synthesis of 1-benzofuran-5-carboxylic acid from methyl benzofuran-5-carboxylate: Methyl benzofuran-5-carboxylate (1.3 g, 7.38 mmol) was dissolved in methanol (51 mL), 5% aqueous sodium hydroxide solution (41 mL) was added, and the mixture was stirred. The reaction mixture was heated to 65 °C and kept at this temperature for 4 hours. After completion of the reaction, the mixture was cooled to room temperature and the methanol was removed by distillation under reduced pressure. The remaining aqueous phase was extracted with dichloromethane and the organic phase was discarded. The aqueous phase was acidified with concentrated hydrochloric acid to pH=1 and then extracted with chloroform. The organic phases were combined, washed with deionized water, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 1.2 g (98% yield) of 1-benzofuran-5-carboxylic acid as a white solid.1H NMR (400 MHz, DMSO-d6) δ 12.9, 8.30, 8.11, 7.92, 7.69, 7.09.

108763-47-9
70 suppliers
$30.00/100mg

90721-27-0
126 suppliers
$22.00/250mg
Yield: 98%
Reaction Conditions:
with sodium hydroxide in methanol;water at 65; for 4 h;
Steps:
1
A stirred mixture of methyl benzofuran-5-carboxylate (1.3 g, 7.38 mmol) in MeOH (51 mL) and sodium hydroxide (41 mL of a 5% aqueous solution) is heated to 65° C. for 4 h. The mixture is cooled to rt, and MeOH is removed in vacuo. The remaining aqueous layer is extracted with CH2Cl2. The CH2Cl2 layer is discarded, and the aqueous layer is acidified to pH=1 with concentrated hydrochloric acid. The aqueous layer is extracted with CHCl3. The organic layer is washed with water, dried (MgSO4), filtered and concentrated in vacuo to afford 1.2 g (98%) of benzofuran-5-carboxylic acid as a white solid. 1H NMR (400 MHz, DMSO-d6) δ12.9, 8.30, 8.11, 7.92, 7.69, 7.09.
References:
Walker, Daniel Patrick;Piotrowski, David W.;Jacobsen, Eric Jon;Acker, Brad A.;Groppi JR., Vincent E. US2003/232853, 2003, A1 Location in patent:Page 37
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

108763-47-9
70 suppliers
$30.00/100mg

90721-27-0
126 suppliers
$22.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

90721-27-0
126 suppliers
$22.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

588702-80-1
50 suppliers
$36.00/1g

90721-27-0
126 suppliers
$22.00/250mg
90843-31-5
150 suppliers
$52.29/0.5g

90721-27-0
126 suppliers
$22.00/250mg