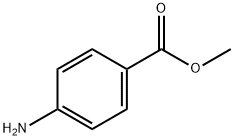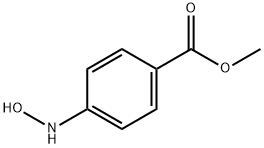
Benzoic acid, 4-(hydroxyamino)-, methyl ester synthesis
- Product Name:Benzoic acid, 4-(hydroxyamino)-, methyl ester
- CAS Number:24226-29-7
- Molecular formula:C8H9NO3
- Molecular Weight:167.16
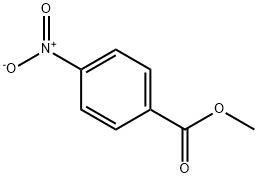
619-50-1
183 suppliers
$17.00/25g

24226-29-7
7 suppliers
inquiry
Yield:24226-29-7 100%
Reaction Conditions:
with 5% rhodium-on-charcoal;hydrazine hydrate monohydrate in tetrahydrofuran at 0;Inert atmosphere;
Steps:
methyl 4-(hydroxyamino)benzoate (S1q)
Under N2 atmosphere, a suspension of methyl 4-nitrobenzoate (5.00 g,0 27.6 mmol, 1.00 equiv) and 5% h/C (159 mg, 0.30 mol% Rh) in THF (300 mL, 0.0920 M) was cooled to 0 °C. Hydrazine monohydrate (1.52 g, 30.4 mmol, 1.20 equiv) was added dropwise. The reaction mixture stirred at 0°C for 5h. The reaction mixture was filtered through a short pad of celite, the celitewas washed with EtOAc and the combined organic5 layers were concentrated in vacuo to afford the title compound as a yellow solid (4.62 g, 27.6 mmol, quant yield). Rf = 0.23 (hexanes/EtOAc 4:1 (v/v) ) . NMR Spectroscopy: XH NMR (500MHz, (CD3)2SO, δ) : 8.95 (s, 1H) , 8.64 (d, u7= 1.5 Hz, 1H) , 7.76 (d, -=8.7 Hz, 2H) , 6.82 (d, J= 8.7 Hz, 2H) , 3.77(s, 3H) . 13C NMR (125 MHz, (CD3)2SO, δ) : 166.2, 156.0,0 130.4, 119.1, 111.2, 51.4. Mass Spectrometry: HRMS (ESI-TOF) (m/z): calcd for CaHioNOa ( [M + H]+), 168.0661, found, 168.0667.
References:
WO2016/57931,2016,A1 Location in patent:Page/Page column 65