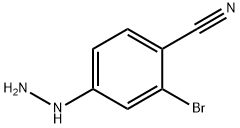
Benzonitrile, 2-broMo-4-hydrazinyl- synthesis
- Product Name:Benzonitrile, 2-broMo-4-hydrazinyl-
- CAS Number:263845-82-5
- Molecular formula:C7H6BrN3
- Molecular Weight:212.05
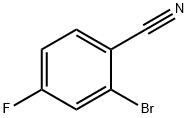
36282-26-5

263845-82-5
Example 4a Preparation of 2-bromo-4-hydrazinobenzonitrile: Anhydrous hydrazine (10 mL) was slowly added to a solution of 2-bromo-4-fluorobenzonitrile (5.00 g, 25.00 mmol) in tetrahydrofuran (THF, 10 mL) through an addition funnel under dry conditions. After the addition was completed, the reaction mixture was stirred at room temperature for 24 hours. Upon completion of the reaction, water (20 mL) was added to the mixture to quench the reaction. The resulting white precipitate was collected by diafiltration and washed well with deionized water. Subsequently, the product was dried in a vacuum oven at 40 °C to afford 2-bromo-4-hydrazinylbenzonitrile (4.92 g, 93% yield) as a white solid. The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3): δ 8.05 (br s, 1H), 7.47 (d, J = 8.7 Hz, 1H), 7.06 (d, J = 2.0 Hz, 1H), 6.72 (dd, J = 8.7, 2.0 Hz, 1H), 4.37 (br s, 2H); ESI MS m/z 212 [M + H]+.

36282-26-5
208 suppliers
$7.00/5g

263845-82-5
49 suppliers
inquiry
Yield:263845-82-5 93%
Reaction Conditions:
with hydrazine in tetrahydrofuran at 20; for 24 h;
Steps:
4.4a
Example 4a Preparation of 2-Bromo-4-hydrazino-benzonitrile To a solution of 2-bromo-4-fluorobenzonitrile (5.00 g, 25.00 mmol) in THF (10 mL) is added anhydrous hydrazine (10 mL) through an addition funnel. After addition, the resulting mixture is stirred at room temperature for 24 h. Water (20 mL) is added to the reaction mixture. A white precipitate is collected by filtration, washed with water, and dried in a vacuum oven at 40° C. to afford 2-Bromo-4-hydrazino-benzonitrile (4.92 g, 93%) as a white solid: 1H NMR (300 MHz, CDCl3) δ8.05 (br s, 1H), 7.47 (d, J=8.7 Hz, 1H), 7.06 (d, J=2.0 Hz, 1H), 6.72 (dd, J=8.7, 2.0 Hz, 1H), 4.37 (br s, 2H); ESI MS m/z 212 [M+H]+.
References:
US2008/119457,2008,A1 Location in patent:Page/Page column 45
57381-49-4
88 suppliers
inquiry
16889-21-7
63 suppliers
$26.00/250mg

263845-82-5
49 suppliers
inquiry
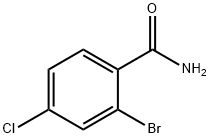
131002-01-2
17 suppliers
$72.00/1g

263845-82-5
49 suppliers
inquiry