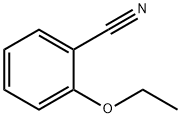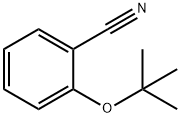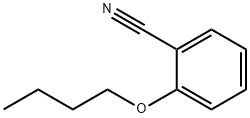
Benzonitrile,2-butoxy- synthesis
- Product Name:Benzonitrile,2-butoxy-
- CAS Number:6609-59-2
- Molecular formula:C11H13NO
- Molecular Weight:175.23
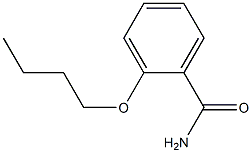
60444-92-0
1 suppliers
inquiry

6609-59-2
5 suppliers
inquiry
Yield:6609-59-2 87%
Reaction Conditions:
Stage #1: 2-butoxybenzamidewith Bromotrichloromethane;triphenylphosphine in dichloromethane; for 0.416667 h;Reflux;
Stage #2: with triethylamine in dichloromethane; for 12 h;Reflux;
Steps:
Typical procedure A to prepare benzonitriles from benzamides
General procedure: BrCCl3 (900 mg, 4.6 mmol) was added to PPh3 (1.22 g, 4.6 mmol) in dry CH2Cl2 (12 mL) and the resulting mixture was stirred at room temperature for 20 min, during which time the solution turned from yellow to red-brownish in colour.Thereafter, 3-chlorobenzamide (1i, 500 mg, 3.2 mmol) was added. Thereaction mixture was heated under reflux for 25 min. Then, dry Et3N (465 mg, 4.6 mmol) was added dropwise with a syringe over 1 min.The reaction mixture was heated under reflux for 12 h. Thereafter,it was cooled to room temperature and added to cold water (50 mL).The mixture was extracted with dichloromethane (2 × 30 mL) and chloroform (30 mL). The combined organic phases were dried over anhydrous MgSO4, concentrated under reduced pressure and subjected to column chromatography on silica gel (CH2Cl2/hexane 4 : 1) to give 3-chlorobenzonitrile (2i, 320 mg, 72%) as a colourless solid.
References:
Jasem, Yosef Al;Barkhad, Mohamed;Khazali, Mona Al;Butt, Hifsa Pervez;El-Khwass, Noha Ashraf;Azani, Mariam Al;Hindawi, Bassam Al;Thiemann, Thies [Journal of Chemical Research,2014,vol. 38,# 2,p. 80 - 84]