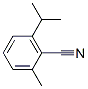
Benzonitrile, 2-methyl-6-(1-methylethyl)- (9CI) synthesis
- Product Name:Benzonitrile, 2-methyl-6-(1-methylethyl)- (9CI)
- CAS Number:786677-15-4
- Molecular formula:C11H13N
- Molecular Weight:159.2276
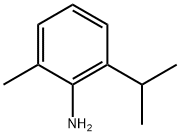
5266-85-3
120 suppliers
$45.00/100mg

786677-15-4
4 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 2-isopropyl-6-methylanilinewith tert.-butylnitrite;CuCN, copper(I) cyanide in dimethyl sulfoxide at 60; for 1 h;
Stage #2: with hydrogenchloride in water;dimethyl sulfoxide at 45; for 0.0833333 h;
Steps:
14.A Example 14; The preparation of 3-Oxo-3H-benzo[d]isothiazole-2-carboxylic acid 2-isopropyl-6-methylbenzamide, A. The preparation of 2-isopropyl-6-methylbenzonitrile
CuCN (7.80 g, 87.2 mmol) was added to a stirred anhydrous DMSO (70 mL) at 60 ° C to form a clear solution, then followed by the addition of tert-butyl nitrite (24.0 mL, 202 mmol) all at once. A solution of 2-isopropyl-6-methylaniline (10.0 g, 67.0 mmol) in anhydrous DMSO (30 mL) was added dropwise, via an addition funnel, to the mixture. After the addition was complete, the reaction mixture was allowed to stir for 1 hr. After being cooled to 45 ° C, the mixture was slowly treated with 5N HCl (100 mL). Five minutes later, the reaction mixture was cooled to ambient temperature before it was extracted with EtOAc/hexane (1 : 1; 500 x 2 mL). The combined organic layers were washed with water (100 mL) and brine (100 mL), dried, concentrated in vacuo, then chromatographed on silica (0-5 % EtOAc in hexane) to give 8.43 g of the crude nitrile 2b. IR (CHCl3) 2220 cm-1; 1H-NMR (CDCl3) δ 1.30 (d, J = 6.9 Hz, 6H), 2.54 (s, 3H), 3.38 (h, J = 6.9 Hz, 1H), 7.13 (d, J = 7.8 Hz, 1H), 7.20 (d, J = 7.8 Hz, 1H), 7.41 (t, J = 7.8 Hz, 1H); ESIMS m/e 160 (M++1).
References:
WO2004/94394,2004,A1 Location in patent:Page 39