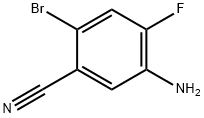
Benzonitrile, 5-amino-2-bromo-4-fluoro- synthesis
- Product Name:Benzonitrile, 5-amino-2-bromo-4-fluoro-
- CAS Number:1613386-27-8
- Molecular formula:C7H4BrFN2
- Molecular Weight:215.02
859855-53-1
111 suppliers
$5.00/250mg

1613386-27-8
5 suppliers
inquiry
Yield:1613386-27-8 62%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 20; for 12 h;
Steps:
Preparation of 5-amino-2-bromo-4-fluorobenzonitrile
3-amino-4-fluorobenzonitrie (9.82 g, 72.1 mmol) was dissolved in acetonitrie to give a pale yellow solution. NBS (13.5g, 75.7 mmol) was added in portions; the reaction mixture tumed brown-blackish but remained a soution. After the completion of the reaction was confirmed by TLC and HPLC, silica gel was added to the reaction mixture and the solvent was evaporated under reduced pressure. The crude product was leaded on a CombiFlash column (330 g) and the product was eluted with hexanes/ethyl acetate (0 - 15% gradient). The clean fractions were combined to give 9.63 g (62%) of a yellowish off-white solid. More product was found in mixed fractions which were discarded. 1H NMR (300 MHz, dmso) 3 7.57 (d, J = 10.9 Hz. 1H). 7.17 (d. J= 8.7 Hz. 1H). 5.85 (s br. 2H); MS (ES) 213/215 (M-H).
References:
WO2014/89112,2014,A1 Location in patent:Paragraph 00330