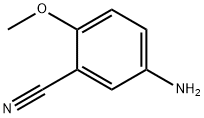
Benzonitrile, 5-amino-2-methoxy- (9CI) synthesis
- Product Name:Benzonitrile, 5-amino-2-methoxy- (9CI)
- CAS Number:214623-57-1
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
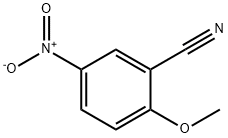
10496-75-0

214623-57-1
General procedure for the synthesis of 5-amino-2-methoxybenzonitrile from 2-methoxy-5-nitrobenzonitrile: 2-methoxy-5-nitrobenzonitrile (1 equiv., 220 mg, 1.23 mmol), methanol (20 mL), and carbon-carrying palladium (10% mass, 10 mg) were added sequentially to a 100 mL round-bottom flask. The flask was connected to a three-way system and the reaction was stirred at room temperature. Evacuation was carried out and then filled with nitrogen, and this cycle was repeated three times, each time the nitrogen was filled for not less than 5 minutes. The reaction was allowed to proceed at room temperature for 30 minutes under hydrogen protection. Upon completion of the reaction, the palladium-carbon catalyst was removed by filtration, the filtrate was concentrated and purified by column chromatography (petroleum ether:ethyl acetate = 5:1) to afford 150 mg of the target product 5-amino-2-methoxybenzonitrile (Compound 7) as a white crystalline solid in 82% yield.

10496-75-0
69 suppliers
inquiry

214623-57-1
82 suppliers
$39.00/250mg
Yield: 82%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20; for 0.5 h;
Steps:
5 5-amino-2-methoxybenzyl cyanide
The 2-methoxy-5-nitrobenzyl cyanide (1eq, 220mg, 1.23mmo1), 20mL of methanol, 10mg of palladium on carbon (10% of mass) were added sequentially in to the 100mL round bottom flask, the bottle connected to the three links, reaction was stirred at room temperature. Vacuum pumping then recharging N2 , repeat this cycle for 3 times , and N2 loading time not less than 5 min. the reaction was allow to react for 30min at room temperature under H2 protection. After the reaction was complete in 30mins , the palladium-carbon was filtered, concentrated, and column chromatography separation (PE: EA = 5: 1) to give 150 mg of product as a white crystalline solid (compound 7). Yield 82%.
References:
Chinese Academy Of Sciences Shanghai Pharmaceutical Institute;Long Yaqiu;Xu Zhongliang;Wang Heyao;Cai Mengxin CN106892871, 2017, A Location in patent:Paragraph 0109; 0112; 0113
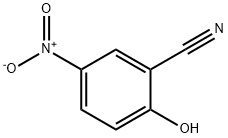
39835-09-1
67 suppliers
inquiry

214623-57-1
82 suppliers
$39.00/250mg
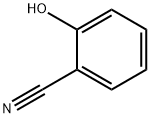
611-20-1
570 suppliers
$6.00/5g

214623-57-1
82 suppliers
$39.00/250mg