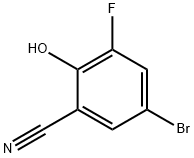
Benzonitrile, 5-bromo-3-fluoro-2-hydroxy- synthesis
- Product Name:Benzonitrile, 5-bromo-3-fluoro-2-hydroxy-
- CAS Number:876918-40-0
- Molecular formula:C7H3BrFNO
- Molecular Weight:216.01
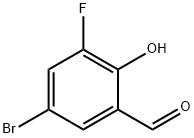
251300-28-4
114 suppliers
$29.00/1g

876918-40-0
23 suppliers
inquiry
Yield:876918-40-0 96%
Reaction Conditions:
with ammonium hydroxide;iodine in tetrahydrofuran;water at 20; for 16 h;
Steps:
6.2 Step 2:
5-bromo-3-fluoro-2-hydroxybenzonitrile
Iodide (1155 mg, 4.56 mmol) was added to a mixture of 5-bromo-3-fluoro-2-hydroxybenzaldehyde (500 mg, 2.29 mmol), ammonium hydroxide (10 mL) and tetrahydrofuran (10 mL), and the mixture was stirred at room temperature for 16 h.
Then sodium thiosulfate (50 mL) was added, and ethyl acetate (100 mL*3) was added for extraction.
The organic phases were combined, washed with saturated brine (100 mL*3), dried over anhydrous sodium sulfate, filtered and concentrated.
The residue was purified by flash chromatography (ethyl acetate/petroleum ether=0%-75%) to give 5-bromo-3-fluoro-2-hydroxybenzonitrile in the form of a yellow solid (480 mg, 96%).
1H NMR (400 MHz, DMSO-d6) δ 7.27-7.34 (m, 2H).
References:
US2020/369676,2020,A1 Location in patent:Paragraph 0851; 0853
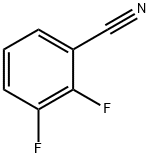
21524-39-0
239 suppliers
$9.00/1g

876918-40-0
23 suppliers
inquiry
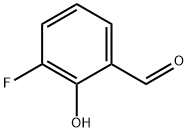
394-50-3
243 suppliers
$9.00/250mg

876918-40-0
23 suppliers
inquiry