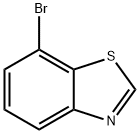
Benzothiazole, 7-bromo- (7CI,8CI) synthesis
- Product Name:Benzothiazole, 7-bromo- (7CI,8CI)
- CAS Number:767-70-4
- Molecular formula:C7H4BrNS
- Molecular Weight:214.08
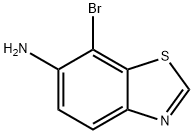
769-20-0

767-70-4
General procedure for the synthesis of 7-bromobenzothiazole from 6-amino-7-bromobenzothiazole: 1. sodium nitrite (0.229 g, 3.3 mmol) was slowly added to a solution of 6-amino-7-bromobenzothiazole (0.380 g, 1.66 mmol, prepared with reference to WO 97/31636) in sulfuric acid (10 mL). 2. The reaction mixture was stirred at room temperature for 20 min. 3. Hypophosphorous acid (10 mL) was added slowly, followed by heating the mixture to 50 °C and reacting overnight. 4. Upon completion of the reaction, the pH was adjusted to 9-10 with sodium carbonate solution, the crude product was collected by filtration and washed with distilled water. 5. The crude product was purified by preparative high performance liquid chromatography (HPLC) to afford 7-bromobenzothiazole (0.265 g, 75% yield) as a white solid. 6. The product was analyzed by LC-MS. 6. The product was analyzed by LC-MS (EI) and showed m/z 213 (M++1).

769-20-0
78 suppliers
$45.00/50mg

767-70-4
86 suppliers
$80.00/50 mg
Yield: 75%
Reaction Conditions:
Stage #1:6-amino-7-bromo-1,3-benzothiazole with sulfuric acid;sodium nitrite at 20; for 0.333333 h;
Stage #2: with hypophosphorous acid at 50;
Stage #3: with sodium carbonate; pH=9 - 10
Steps:
30
Example 30; 7-Bromo-benzothiazoleSodium nitrite (0.229 g, 3.3 mmol) was added to a solution of 7-bromo-benzothiazol-6-ylamine (0.380 g, 1.66 mmol, described in WO 97/31636) in sulfuric acid (10 mL) and the resulting mixture was stirred at room temperature for 20 min. Hypophosphorous acid (10 mL) was added and the mixture was heated at 50° C. overnight. The pH was adjusted to 9-10 by addition of sodium carbonate, and the crude product was removed by filtration and washed with water. Purification by prep-HPLC gave 0.265 g (75% yield) of the title compound as a white solid: LC-MS (EI) m/z 213 (M++1).
References:
AstraZeneca AB US2011/98292, 2011, A1 Location in patent:Page/Page column 1
533-30-2
201 suppliers
$7.00/250mg

767-70-4
86 suppliers
$80.00/50 mg
1123-55-3
87 suppliers
$56.00/100 mg

767-70-4
86 suppliers
$80.00/50 mg