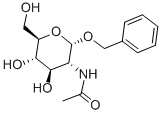
BENZYL 2-ACETAMIDO-2-DEOXY-ALPHA-D-GLUCOPYRANOSIDE synthesis
- Product Name:BENZYL 2-ACETAMIDO-2-DEOXY-ALPHA-D-GLUCOPYRANOSIDE
- CAS Number:13343-62-9
- Molecular formula:C15H21NO6
- Molecular Weight:311.33
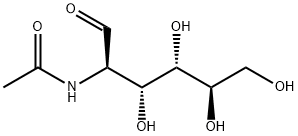
7512-17-6

100-51-6

13343-62-9
GENERAL STEPS: N-acetylglucosamine (6.40 g, 28.9 mmol) was dissolved in benzyl alcohol (50 mL, 480 mmol) and concentrated aqueous hydrochloric acid solution (3.0 mL) was added. The reaction mixture was stirred at 90°C for 3 hours. After completion of the reaction, the crude product was cooled to room temperature, poured into ether (500 mL) and crystallized at 4°C for 18 hours. The crystallized product was collected by filtration and washed with petroleum ether. Purification by silica gel column chromatography (eluent: dichloromethane/methanol, 95:5 to 88:12, v/v) afforded benzylglycoside 2 (6.67 g, 21.4 mmol, 74% yield) as a white, foamy solid. rf = 0.25 (dichloromethane/methanol, 90:10, v/v); melting point 182-184°C; [α]D17.8 = +222 (c 0.1, methanol); IR (thin film) νmax 3298, 3092, 2938, 2901, 2844, 1648, 1552, 1497, 1455, 1375, 1309, 1230, 1156, 1093, 1047, 778, 732, 695 cm?1; 1H NMR (500 MHz, CD3OD) δ 7.40-7.25 (m, 5H, aromatic H), 4.85 (d, 1H, J = 3.6 Hz, H-1′), 4.74 (d, 1H, J = 12.0 Hz, H-1a), 4.49 (d, 1H, J = 12.0 Hz, H-1b), 3.89 (dd, 1H, J = 3.6, 10.8 Hz, H-2′), 3.83 ( dd, 1H, J = 1.6, 11.4 Hz, H-6a′), 3.73-3.64 (m, 3H, H-6b′, H-3′, H-4′), 3.36 (dd, 1H, J = 9.6 Hz, H-5′), 1.95 (s, 3H, acetyl CH3); 13C NMR (125 MHz, CD3OD) δ 173.6 (C= O, acetyl), 139.0 (aromatic quaternary carbon), 129.4, 129.3, 128.8 (aromatic CH), 97.5 (C-1′), 74.1 (C-4′), 72.7 (C-3′), 72.5 (C-5′), 70.1 (C-1), 62.7 (C-6′), 55.4 (C-2′), 22.5 (acetyl CH3 ); HRMS (ESI) m/z [C15H22NO6]+ Calculated value: 312.1442, measured value: 312.1446.

7512-17-6
668 suppliers
$5.00/1g

100-51-6
1435 suppliers
$5.00/100g

13343-62-9
92 suppliers
$26.00/1g
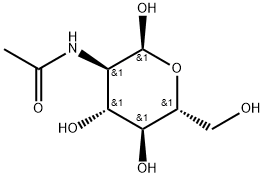
10036-64-3
92 suppliers
$25.00/1g

100-51-6
1435 suppliers
$5.00/100g

13343-62-9
92 suppliers
$26.00/1g