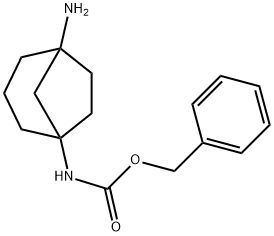
Benzyl (5-Aminobicyclo[3.2.1]Octan-1-Yl)Carbamate synthesis
- Product Name:Benzyl (5-Aminobicyclo[3.2.1]Octan-1-Yl)Carbamate
- CAS Number:1383973-53-2
- Molecular formula:C16H22N2O2
- Molecular Weight:274.36
![Bicyclo[3.2.1]octane-1-carboxylic acid, 5-[[(phenylmethoxy)carbonyl]amino]-](/CAS/20210305/GIF/1383973-52-1.gif)
1383973-52-1
0 suppliers
inquiry
![Benzyl (5-Aminobicyclo[3.2.1]Octan-1-Yl)Carbamate](/CAS/20200401/GIF/1383973-53-2.gif)
1383973-53-2
26 suppliers
inquiry
Yield:1383973-53-2 42%
Reaction Conditions:
Stage #1: 5-(benzyloxycarbonylamino)bicyclo[3.2.1]octane-1-carboxylic acidwith diphenyl phosphoryl azide;triethylamine in toluene at 18 - 25; for 4 h;Inert atmosphere;Reflux;Curtius Rearrangement;
Stage #2: with potassium trimethylsilonate in tetrahydrofuran;toluene at 0 - 25; for 1 h;
Stage #3: with citric acid in tetrahydrofuran;toluene;
Steps:
3.7
Step 7: Benzyl 5-aminobicyclo|3.2.1 |octan-I-ylcarbamateA mixture of 5-(benzyloxycarbonylamino)bicyclo[3.2.1 ]octane- l -carboxylic acid (8.5 g, 28.0 mmol), diphenylphosphonic azide (9.3 g, 33.8 mmol) and triethylamine (5 mL, 68 mmol) in toluene ( 150 mL) was stirred at room temperature for one hour, and then refluxed for three hours. After cooled to 0°C, a solution of TMSOK (10.7 g, 83.6 mmol) in THF (85 mL) was added. The reaction mixture was wanned to room temperature and stirred for l h, and then quenched with 5% citric acid (20 mL) and concentrated under reduced pressure. The residue was treated with aq. H'Cl (2 N, 200 mL) at 0°C. The resulting mixture was extracted with ethyl acetate (3 x 100 mL). The aqueous phase was adjusted to pH 9~10 with Na2C03 and extracted with dichloromethane/methanol ( 10/1 , 3 x 150 mL). The combined organic layer was washed with water and brine, dried over sodium sulfate and concentrated under reduced pressure to afford 5.13 g (42%) of benzyl 5-aminobicyclo[3.2.1 ]octan- l -ylcarbamate as a yellow solid. ES1-MS m/z: 275 ( ++ l).
References:
WO2012/88365,2012,A1 Location in patent:Page/Page column 32-33
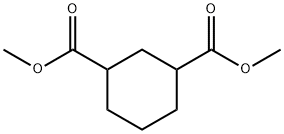
62638-06-6
69 suppliers
$45.00/50mg
![Benzyl (5-Aminobicyclo[3.2.1]Octan-1-Yl)Carbamate](/CAS/20200401/GIF/1383973-53-2.gif)
1383973-53-2
26 suppliers
inquiry

100-51-6
1456 suppliers
$5.00/100g
![Benzyl (5-Aminobicyclo[3.2.1]Octan-1-Yl)Carbamate](/CAS/20200401/GIF/1383973-53-2.gif)
1383973-53-2
26 suppliers
inquiry
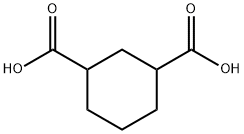
3971-31-1
150 suppliers
$6.00/1g
![Benzyl (5-Aminobicyclo[3.2.1]Octan-1-Yl)Carbamate](/CAS/20200401/GIF/1383973-53-2.gif)
1383973-53-2
26 suppliers
inquiry
![bicyclo[3.2.1]octane-1,5-dicarboxylic acid MonoMethyl ester](/CAS/GIF/110371-27-2.gif)
110371-27-2
19 suppliers
inquiry
![Benzyl (5-Aminobicyclo[3.2.1]Octan-1-Yl)Carbamate](/CAS/20200401/GIF/1383973-53-2.gif)
1383973-53-2
26 suppliers
inquiry