
.beta.-D-ribo-Hexopyranose, 1,6-anhydro-3-deoxy- synthesis
- Product Name:.beta.-D-ribo-Hexopyranose, 1,6-anhydro-3-deoxy-
- CAS Number:29514-08-7
- Molecular formula:C6H10O4
- Molecular Weight:146.14
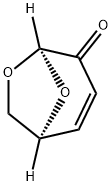
37112-31-5
128 suppliers
$124.00/250mg

29514-08-7
1 suppliers
inquiry

29514-09-8
1 suppliers
inquiry
Yield:29514-09-8 75% ,29514-08-7 15%
Reaction Conditions:
Stage #1: levoglucosenonewith water;triethylamine at 20; for 2 h;Michael Addition;
Stage #2: with sodium tetrahydroborate;water for 1 h;Overall yield = 90 %;
Steps:
(1R,2S,4S,5R)-6,8-Dioxabicyclo[3.2.1]octane-2,4-diol(6) and (1R,2S,4R,5R)-6,8-dioxabicyclo[3.2.1]octane-2,4-diol (6b)
A solution of levoglucosenone (1) (1.00 g,7.93 mmol) and triethylamine (0.55 ml, 3.96 mmol) in H2O(60 ml) was stirred at room temperature for 2 h. The mixture was then treated with 98% NaBH4 (0.31 g,7.93 mmol) and stirred for approximately 1 h. The excess of NaBH4 was decomposed with AcOH (3 ml). The solvent was removed by evaporation on rotary evaporator, the residue (mixture of alcohols 6a,b) was separated chromatographically, eluent EtOAc
References:
Sharipov, Bulat T.;Davidova, Anna N.;Ryabova, Alena S.;Galimzyanova, Nailya F.;Valeev, Farid A. [Chemistry of Heterocyclic Compounds,2019,vol. 55,# 1,p. 31 - 37][Khim. Geterotsikl. Soedin.,2019,vol. 55,# 1,p. 31 - 37,7]

26922-85-0
0 suppliers
inquiry

29514-08-7
1 suppliers
inquiry
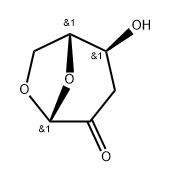
58238-45-2
0 suppliers
inquiry

29514-08-7
1 suppliers
inquiry
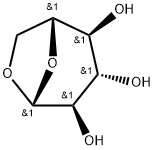
498-07-7
272 suppliers
$11.00/250mg

29514-08-7
1 suppliers
inquiry
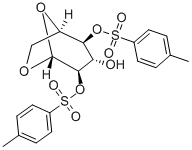
20204-80-2
18 suppliers
$89.50/50mg

29514-08-7
1 suppliers
inquiry