
bicyclo[2.2.2]octane-2,6-dione synthesis
- Product Name:bicyclo[2.2.2]octane-2,6-dione
- CAS Number:38231-60-6
- Molecular formula:C8H10O2
- Molecular Weight:138.16
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![bicyclo[2.2.2]octane-2,6-dione](/CAS/20180629/GIF/38231-60-6.gif)
38231-60-6
2 suppliers
inquiry
Yield: 60%
Reaction Conditions:
with acetic acid at 100; for 1 h;Inert atmosphere;
Steps:
5
(Synthesis Example 5) Synthesis of DION [Show Image][Show Image] DMON (107 g) was added to acetonitrile (300 mL). While this mixture was being stirred at room temperature in a nitrogen stream, tributylethylammonium chloride (106 g) and lithium carbonate (48 g) were added thereto. After the resultant mixture was continuously stirred for 20 minutes, CHNON (40 g) was added dropwise thereto, and this mixture was stirred for 30 hours at room temperature in a nitrogen stream. Ethyl acetate (700 mL) and water (700 mL) were further added to the resultant liquid reaction mixture, and this mixture was stirred. Thereafter, the pH of the liquid reaction mixture was adjusted to 1-2 with 11-N aqueous hydrogen chloride solution. Subsequently, the reaction mixture was subjected to liquid separation, and the organic layer was dried with MgSO4, concentrated to cause crystallization, and then subjected to filtration and washing with methanol. Thus, 85 g (yield, 85%) of DMOHDION (purity, 97%) was obtained. Subsequently, polyphosphoric acid (PPA) (63 g) and acetic acid (55 g) were added to the DMOHDION (85 g), and the mixture was stirred for 60 minutes in a 100°C nitrogen stream. After the mixture was returned to room temperature, saturated aqueous sodium chloride solution (160 mL) which had been cooled was added thereto. The resultant mixture was extracted with toluene, and the extract was washed with saturated aqueous sodium hydrogen carbonate solution. The extract was further dried with sodium sulfate and concentrated. Thus, 36 g (yield, 60%) of DION (purity, 95%) was obtained.(Results of NMR Analysis) Compound DION: 1H NMR (CDCl3, 400 MHz) δ 1.75-1.9 (m, 2H), 2.05-2.2 (m, 2H), 2.3-2.7 (m, 5H), 3.1-3.25 (s, 1H)
References:
Mitsubishi Chemical Corporation EP2495229, 2012, A1 Location in patent:Page/Page column 63-64
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![bicyclo[2.2.2]octane-2,6-dione](/CAS/20180629/GIF/38231-60-6.gif)
38231-60-6
2 suppliers
inquiry
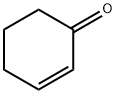
930-68-7
266 suppliers
$9.00/1g
![bicyclo[2.2.2]octane-2,6-dione](/CAS/20180629/GIF/38231-60-6.gif)
38231-60-6
2 suppliers
inquiry
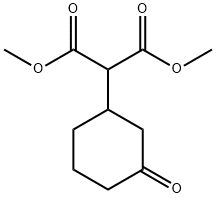
33646-18-3
8 suppliers
inquiry
![bicyclo[2.2.2]octane-2,6-dione](/CAS/20180629/GIF/38231-60-6.gif)
38231-60-6
2 suppliers
inquiry