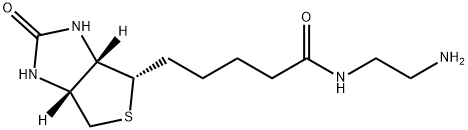
biotinylamidoethylacetamide synthesis
- Product Name:biotinylamidoethylacetamide
- CAS Number:111790-37-5
- Molecular formula:C12H22N4O2S
- Molecular Weight:286.39
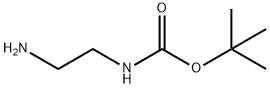
57260-73-8
497 suppliers
$5.00/5g
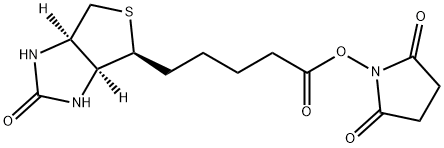
35013-72-0
393 suppliers
$12.00/100mg

111790-37-5
94 suppliers
$50.00/25 mg
Yield: 78%
Reaction Conditions:
Stage #1:N-BOC-1,2-diaminoethane;biotin N-Hydroxysuccinimide ester in N,N-dimethyl-formamide at 20;
Stage #2: with hydrogenchloride in 1,4-dioxane;methanol
Steps:
1 iV-(2-aminoethyl)-5-(2-oxo-l,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl)pentanamide (22)
Neat tert-butyl Ar-(2-aminoethyl)carbamate was added to a suspension of NHS-ester 21 in anhydrous DMF (8.0 mL) and the reaction stirred at rt overnight. Upon consumption of starting material EtzO was flowed in the mixture chilled to -20 °C overnight. The resultant precipitate was collected via vacuum filtration and dried under suction to yield Boc-protected amine (1.18 g, 78%) as a white powder. The intervening carbamate was stirred in MeOH containing 4M HC1 to provide the amine after evaporation of the solvent, which was taken on without further purification. lH NMR (500 MHz, DMSO-d6) d 6.45 (s, 1H), 6.39 (s, 1H), 4.30 (dd, J= 7.7, 5.1 Hz, 1H), 4.13 (ddd,J= 7.8, 4.4, 1.9 Hz, 1H), 3.28 (q, J= 6.2 Hz, 2H), 3.10 (ddd,J= 8.6, 6.1, 4.4 Hz, 1H), 2.86 - 2.78 (0269) (m, 3H), 2.57 (d, J= 12.4 Hz, 1H), 2.10 (t, J= 7.5 Hz, 2H), 1.65 - 1.41 (m, 4H), 1.39 - 1.22 (m, 2H). 13C NMR (125 MHz, DMSO-d6) d 172.78, 162.75, 61.05, 59.22, 55.42, 38.59, 36.39, 35.16, 28.26, 28.08, 25.03.
References:
THE UNIVERSITY OF CHICAGO WO2021/55960, 2021, A1 Location in patent:Page/Page column 73; 81-82

35013-72-0
393 suppliers
$12.00/100mg
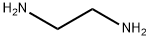
107-15-3
0 suppliers
$11.19/25ML

111790-37-5
94 suppliers
$50.00/25 mg
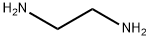
107-15-3
0 suppliers
$11.19/25ML
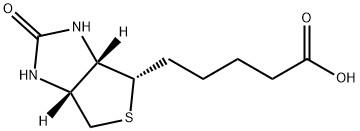
58-85-5
1055 suppliers
$5.00/10mg

111790-37-5
94 suppliers
$50.00/25 mg

225797-46-6
35 suppliers
$140.00/50 mg

111790-37-5
94 suppliers
$50.00/25 mg
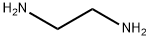
107-15-3
0 suppliers
$11.19/25ML
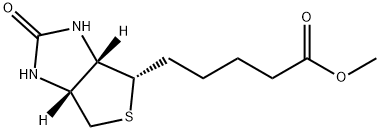
608-16-2
52 suppliers
$35.00/100mg

111790-37-5
94 suppliers
$50.00/25 mg