
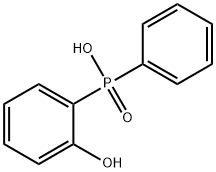
bis(2-hydroxyphenyl)phenylphosphine oxide synthesis
- Product Name:bis(2-hydroxyphenyl)phenylphosphine oxide
- CAS Number:112122-94-8
- Molecular formula:C18H15O3P
- Molecular Weight:310.29
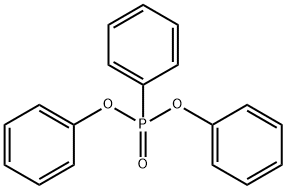
3049-24-9
36 suppliers
$15.00/5g

112122-94-8
1 suppliers
inquiry
Yield:112122-94-8 79%
Reaction Conditions:
with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -75 - 20; for 6 h;Inert atmosphere;
Steps:
Bis(2-hydroxyphenyl)phenyl-λ5-phosphanone (13)
To a vigorously stirred solution of freshly distilled pure and anhyd i-Pr2NH (20 mL, 14.5 g, 0.14 mol) in pure and anhyd THF (30 mL) was added 2.5 M n-BuLi in hexanes (57 mL, 0.14 mol) at -75 °C under argon over 30 min, and stirring was continued for another 30 min. After the LDA solution had formed, diphenyl phenylphosphonate (12; 11.1 g, 35.8 mmol) dissolved in pure and anhyd THF (30 mL) was added over 60 min at -75 °C. The resulting mixture was stirred for 60 min, then it was allowed to warm up to rt and stirred for 4 h. After completion of the reaction, the mixture was added in small portions to a vigorously stirred mixture of CH2Cl2 (300 mL), sat. aq NH4Cl (240 mL), and ice (100 g). The organic phase was separated and the aqueous one was extracted with CH2Cl2 (3 × 100 mL). The combined organic phases were washed with H2O (200 mL), dried (MgSO4), filtered, and the solvent was removed. The crude product was purified by recrystallization from toluene to give 13 (8.77 g, 79%) as a white powder. The product had the same physical properties and spectroscopic data as reported in the literature.
References:
Szabó-Szentjóbi, Hajnalka;Majoros, István;Márton, Anna;Leveles, Ibolya;Vértessy, Beáta G.;Dékány, Miklós;Tóth, Tünde;Huszthy, Péter [Synthesis,2020,vol. 52,# 19,p. 2870 - 2882]

1091-27-6
1 suppliers
inquiry
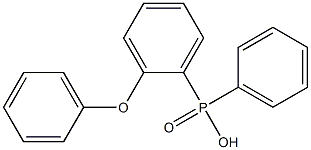
1093-62-5
0 suppliers
inquiry
94407-54-2
1 suppliers
inquiry

112122-94-8
1 suppliers
inquiry