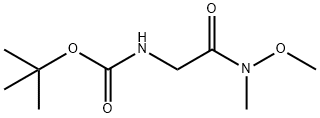
BOC-GLYCINE N,O-DIMETHYLHYDROXAMIDE synthesis
- Product Name:BOC-GLYCINE N,O-DIMETHYLHYDROXAMIDE
- CAS Number:121505-93-9
- Molecular formula:C9H18N2O4
- Molecular Weight:218.25
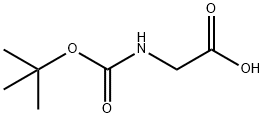
4530-20-5
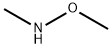
1117-97-1

121505-93-9
To a stirred solution of (tert-butoxycarbonyl)glycine (5.0 g, 28.57 mmol) and triethylamine (4.1 mL, 31.42 mmol) in dichloromethane (80 mL) was added N,O-dimethylhydroxylamine hydrochloride (3.0 g, 31.42 mmol). Subsequently, the reaction mixture was stirred at room temperature for 12 hours by dropwise addition of a solution of N,N'-dicyclohexylcarbodiimide (6.5 g, 31.42 mmol) in dichloromethane (20 mL). The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction mixture was filtered through diatomaceous earth and washed with dichloromethane. The filtrate was concentrated under reduced pressure to give the crude product, which was purified by silica gel column chromatography using 10% ethyl acetate/hexane as eluent to give the final N-BOC-glycine-N'-methoxy-N'-methylamide (6.0 g, 97.0%) as an off-white solid.1H NMR (400 MHz, CDCl3): δ 5.27 (broad single peak, 1H), δ 4.08 (broad single peak, 2H), 3.72 (single peak, 3H), 3.21 (single peak, 3H), 1.46 (single peak, 9H).
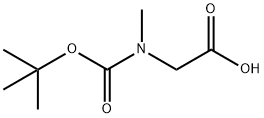
13734-36-6
307 suppliers
$11.19/5G

6638-79-5
594 suppliers
$6.00/25g

121505-93-9
144 suppliers
$6.00/1g
Yield: 95%
Reaction Conditions:
Stage #1:N-(tert-butoxycarbonyl)sarcosine with 1,1'-carbonyldiimidazole in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Stage #2:N,O-dimethylhydroxylamine*hydrochloride in tetrahydrofuran at 20;
Steps:
3.1d tert-Butyl (2-(methoxy(methyl)amino)-2-oxoethyl)(methyl)carbamate
To a solution of 2-((tert-butoxycarbonyl)(methyl)amino)acetic acid (25.0 g, 132.0 mmol) in 300 mL of anhydrous THF under N2 atmosphere was added CDI (1.2 eq; 25.7 g; 159.0 mmol) portionwise over 10 min. The reaction mixture was stirred at room temperature for 2 h and N,O-dimethylhydroxylamine hydrochloride (1.2 eq.; 15.5 g; 159 mmol) and Et3N (1.2 eq; 22.0 mL; 159.0 mmol) were added. The resolution was stirred overnight at room temperature. The precipitated salts were filtered off and the filtrate was concentrated. The residue was diluted with H2O (300 mL) and extracted with EtOAc (3×100 mL). Combined organic phases were washed with 10% KHSO4 (3×50 mL), saturated NaHCO3 (50 mL), water and brine, then dried over MgSO4 and concentrated. This gave tert-Butyl (2-(methoxy(methyl)amino)-2-oxoethyl)(methyl)carbamate as a colorless oil (29 g, 95%). 1H-NMR spectrum in DMSO-d6 indicates a mixture of rotamers (1:1). 1H NMR (DMSO-d6) δ: 4.08 (s, 1H), 4.07 (s, 1H), 3.68 (s, 1.5H), 3.66 (s, 1.5H), 3.10 (s, 1.5H), 3.09 (s, 1.5H), 2.82 (s, 1.5H), 2.78 (s, 1.5H), 1.39 (s, 4.5H), 1.33 (s, 4.5H). MS 132 (MH+).
References:
SENOMYX, INC.;Chumakova, Lyudmyla;Patron, Andrew;Priest, Chad;Karanewsky, Donald;Kimmich, Rachel;Boren, Brant Clayton;Hammaker, Jeffrey Robert;Chumakov, Volodymyr;Zhao, Wen;Noncovich, Alain;Ung, Jane US2015/376136, 2015, A1 Location in patent:Paragraph 0866

4530-20-5
569 suppliers
$5.00/5 g

6638-79-5
594 suppliers
$6.00/25g

121505-93-9
144 suppliers
$6.00/1g

4530-20-5
569 suppliers
$5.00/5 g

121505-93-9
144 suppliers
$6.00/1g

6638-79-5
594 suppliers
$6.00/25g

121505-93-9
144 suppliers
$6.00/1g