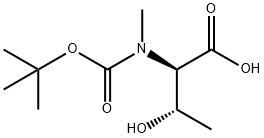
Boc-N-Me-D-Thr-OH synthesis
- Product Name:Boc-N-Me-D-Thr-OH
- CAS Number:170872-87-4
- Molecular formula:C10H19NO5
- Molecular Weight:233.26
226067-37-4
12 suppliers
inquiry

170872-87-4
20 suppliers
inquiry
Yield:170872-87-4 96%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol at 60;
Steps:
41.2 Step 2.
Step 2.
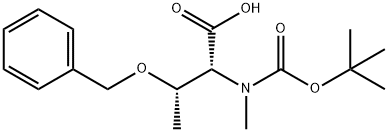
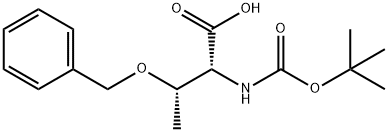
Preparation of (2R,3S)-2-[tert-butoxycarbonyl-(methyl)-amino]-3-hydroxy-butanoic acid
(2R,3S)-3-Benzyloxy-2-[tert-butoxycarbonyl-(methyl)-amino]-butanoic acid (0.5 g, 1.55 mmol) was dissolved in abs. EtOH (100 mL).
The solution was passed through the H-Cube hydrogenator flow reactor provided with a 10% Pd/C cartridge [flow rate=1.0 mL/min, P=1.0 bar, T=60° C.].
The hydrogenated solution was concentrated to dryness to afford title compound (0.35 g, 96%), as a mixture of two rotamers (1:1.3 ratio), as a colorless oil. 1H NMR (DMSO-d6) δ 1.06-1.12 (m, 6H), 1.36 (s, 9H, minor rotamer), 1.40 (s, 9H, major rotamer), 2.86 (s, 3H, minor rotamer), 2.89 (s, 3H, major rotamer), 4.15-4.27 (m, 2H), 4.33 (m, 1H, minor rotamer), 4.47 (d, J=5.3 Hz, 1H, major rotamer).
References:
US9353075,2016,B2 Location in patent:Page/Page column 119
69355-99-3
142 suppliers
$12.00/250mg

170872-87-4
20 suppliers
inquiry