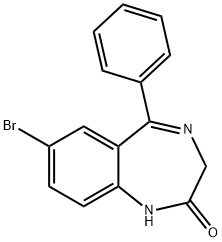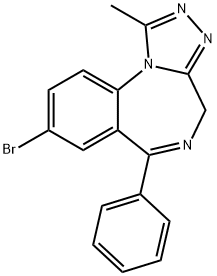
Bromazolam synthesis
- Product Name:Bromazolam
- CAS Number:71368-80-4
- Molecular formula:C17H13BrN4
- Molecular Weight:353.22
A solution of 1 (1 g, 3.07 mmol of 7-bromo-5-phenyl-1,4-benzodiazepine-2-one) in dry THF (20 mL) was cooled in an ice-water bath and a 60% dispersion of sodium hydride (152.2 mg) was added in one portion. After 20 minutes, di-4-morpholinylphosphinic chloride (943.9 mg, 4.76 mmol) was added at 0° C. and this was stirred for 30 minutes and allowed to warm to room temperature (Ning, R Y., et al., (1976) J Org Chem 41: 2724-2727). The mixture was stirred for 1.5 hours. To this mixture was then added a solution of acetylhydrazide (521.9 mg, 7.14 mmol) in dry butanol (5 mL) and stirring was continued at room temperature for 10 min. The solvents were evaporated and the residue was dissolved in butanol (10 mL) and heated to reflux for 5 hours. Butanol was removed under reduced pressure and the residue was partitioned between CH2Cl2 (50 mL) and water (50 mL). The water layer was extracted by CH2Cl2 (3×30 mL). The combined organic layer was washed by brine (30 mL). The organic layer was dried (Na2SO4) and the solvent was removed under vacuum. The residue was purified by flash chromatography (silica gel) to provide pure 8 [539.5 mg (40% yield)] as a white solid.
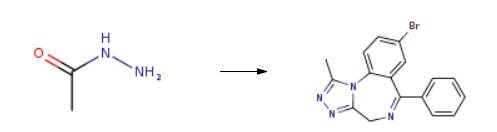
1068-57-1
411 suppliers
$6.00/5g

71368-80-4
0 suppliers
$71.00/1mg
Yield:71368-80-4 40%
Reaction Conditions:
Stage #1: 7-bromo-5-phenyl-1,3-dihydro-2H-benzo[e][1,4]diazepin-2-onewith sodium hydride in tetrahydrofuran at 0; for 0.333333 h;
Stage #2: with di-morpholin-4-yl-phosphinic acid chloride in tetrahydrofuran at 0 - 20; for 2 h;
Stage #3: acetic acid hydrazide in tetrahydrofuran;butan-1-ol at 20; for 0.166667 h;
Steps:
8-Bromo-1-methyl-6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepine 8.; 3 A solution of 1 (1 g, 3.07 mmol of 7-bromo-5-phenyl-1,4-benzodiazepine-2-one) in dry THF (20 mL) was cooled in an ice-water bath and a 60% dispersion of sodium hydride (152.2 mg) was added in one portion. After 20 minutes, di-4-morpholinylphosphinic chloride (943.9 mg, 4.76 mmol) was added at 0° C. and this was stirred for 30 minutes and allowed to warm to room temperature (Ning, R Y., et al., (1976) J Org Chem 41: 2724-2727). The mixture was stirred for 1.5 hours. To this mixture was then added a solution of acetylhydrazide (521.9 mg, 7.14 mmol) in dry butanol (5 mL) and stirring was continued at room temperature for 10 min. The solvents were evaporated and the residue was dissolved in butanol (10 mL) and heated to reflux for 5 hours. Butanol was removed under reduced pressure and the residue was partitioned between CH2Cl2 (50 mL) and water (50 mL). The water layer was extracted by CH2Cl2 (3×30 mL). The combined organic layer was washed by brine (30 mL). The organic layer was dried (Na2SO4) and the solvent was removed under vacuum. The residue was purified by flash chromatography (silica gel) to provide pure 8 [539.5 mg (40% yield)] as a white solid: mp 268.5-270° C.; IR (KBr) 2358, 1607, 1538, 1484, 1311, 1000, 801, 697 cm-1; 1H NMR (CDCl3) δ 2.82(s, 3H), 4.11(d,1H, J=12.8 Hz), 5.49 (d,1H, J=12.8 Hz), 7.21-7.68(m, 7H), 7.75 (dd, 1H, J=0.58 Hz and 1.5 Hz); MS (EI) m/e (relative intensity) 354 (34), (M+, 16), 352 (34), 325(33), 323 (34), 273 (63), 245 (31), 232 (19), 204 (100), 183(23), 177 (36), 151 (24). Anal. Calcd. for C17H13BrN4: C, 57.81; H, 3.71; N, 15.86. Found C, 57.57; H, 3.64: N, 15.70.
References:
US2006/3995,2006,A1 Location in patent:Page/Page column 29-30