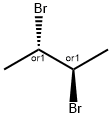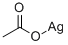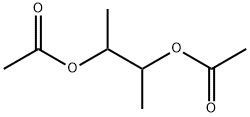
butane-2,3-diyl diacetate synthesis
- Product Name:butane-2,3-diyl diacetate
- CAS Number:1114-92-7
- Molecular formula:C8H14O4
- Molecular Weight:174.19

624-64-6
76 suppliers
$195.00/50 g

1114-92-7
21 suppliers
inquiry
Yield: 23%
Reaction Conditions:
with acetic anhydride;iodosylbenzene diacetate
Steps:
V EXAMPLE V
EXAMPLE V To demonstrate the effectiveness of the reaction system of this invention for the oxidation of a monoolefin, a one-liter stainless steel autoclave was charged with 300 ml of acetic anhydride, 6.4 grams (20 mmoles) of iodosobenzene diacetate and 53 grams (946.4 mmoles) of trans-2-butene. The reactor was pressured to 30 psig with oxygen and heated to 140° C. The reaction vessel was pressured from time to time with oxygen as in the earlier runs up to about 200 psig during about 2 hours of the three and one-half hours reaction period. At the conclusion of the reaction, the autoclave was vented and the reaction mixture transferred to a distillation flask utilizing a little acetic anhydride as a rinsing solvent for the reaction vessel. The mixture was distilled through a 3/4" (1.91 cm)*15" (39 cm) column packed with 6 millimeter Raschig rings. Four fractions were collected during the distillation procedure. Analysis by gas-liquid phase chromatography of fractions 3 to 4 indicated that 217.2 mmoles of 2,3-diacetoxy butane were obtained for a yield of 23 percent based on the starting trans-2-butene.
References:
Phillips Petroleum Company US4221917, 1980, A

141-78-6
665 suppliers
$20.50/250ml

1114-92-7
21 suppliers
inquiry
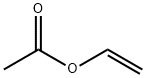
108-05-4
590 suppliers
$4.00/1000g

1114-92-7
21 suppliers
inquiry