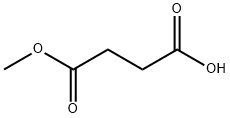
Butanedioic acid, bromo-, 1-methyl ester synthesis
- Product Name:Butanedioic acid, bromo-, 1-methyl ester
- CAS Number:98298-19-2
- Molecular formula:C5H7BrO4
- Molecular Weight:211.01
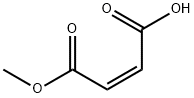
3052-50-4
141 suppliers
$5.00/5g

98298-19-2
7 suppliers
inquiry
Yield: 81.8%
Reaction Conditions:
with hydrogen bromide in acetic acid at 0 - 5;
Steps:
1.A Example 1; Preparation of methyl 2-(3-chloro-2-pyridinyl)-5-oxo-3-pyrazolidinecarboxylate (Formula I wherein R1 is methyl, R2a and R2b are H and L is 3-chloro-2-pyridinyl); Step A: Preparation of 1-methyl hydrogen bromobutanedioate
Methyl hydrogen (2Z)-2-BUTENDIOATE (also known as the monomethyl ester of maleic acid) (50 g, 0. 385 mol) was added dropwise to a solution of hydrogen bromide in acetic acid (141.43 g, 33%, 0.577 mol) at 0 °C over 1 h. The reaction mixture was stored at about 5 °C overnight. The solvent was then removed under reduced pressure. Toluene (100 ML) was added, and the mixture was evaporated under reduced pressure. The process was repeated three times using more toluene (3 x 100 mL). Then toluene (50 ML) was added, and the mixture was cooled TO-2 °C. Hexanes (50 mL) was added dropwise to the mixture. When the addition was complete the mixture was stirred about 30 minutes while the product crystallized. The product was then isolated by filtration and dried in vacuo to provide the title compound as a white solid (63.37 g, 81.8% yield). A sample recrystallized from toluene/hexanes melted at 38-40 °C. IR (nujol): 1742,1713, 1444,1370, 1326,1223, 1182,1148, 1098,996, 967,909, 852 CM-L. 1H NMR (CDC13) 8 4.57 (X of ABX pattern, J = 6.1, 8.9 HZ, 1H), 3.81 (s, 3H), 3.35 (1/2 of AB in ABX pattern, J = 8.8, 17.7 Hz, 1H), 3.05 (1/2 of AB in ABX pattern, J = 6.1, 17.8
References:
E.I. DUPONT DE NEMOURS AND COMPANY WO2004/87689, 2004, A1 Location in patent:Page 20-21