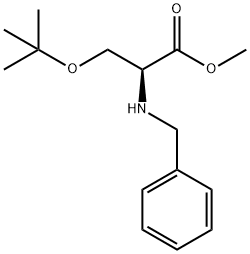
BZL-SER(TBU)-OME HCL synthesis
- Product Name:BZL-SER(TBU)-OME HCL
- CAS Number:670278-82-7
- Molecular formula:C15H23NO3
- Molecular Weight:265.35

100-52-7
952 suppliers
$20.37/250g
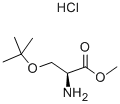
17114-97-5
156 suppliers
$8.00/1g

670278-82-7
32 suppliers
$60.00/100mg
Yield:670278-82-7 52%
Reaction Conditions:
Stage #1: benzaldehyde;O-tert-butyl-L-serine methyl ester hydrochloridewith sodium cyanoborohydride;acetic acid in tetrahydrofuran;methanol at 20; for 48 h;
Stage #2: with water;sodium hydrogencarbonateCooling with ice;
Steps:
24.1
Step 1: methyl N-benzyl-O-tert-butyl-L-serinate Methyl O-tert-butyl-L-serinate hydrochloride (12 g, 56.7 mmol) was dissolved in methanol (120 ml), acetic acid (6.50 ml, 114 mmol), benzaldehyde (6.25 ml, 61.8 mmol), and sodium cyanoborohydride (1 M tetrahydrofuran solution, 75 ml) were added and the resulting mixture was stirred at room temperature for 48 hours. The reaction mixture was concentrated, diluted with water, and neutralized with sodium bicarbonate under ice cooling, followed by extraction with chloroform. The organic layer was dried over anhydrous magnesium sulfate, then the drying agent was removed by filtration and the solvent was concentrated under reduced pressure. The residue obtained was purified by silica gel chromatography [ethyl acetate:n-hexane = 1:4 (v/v)] to give the title compound (7.75 g, 52%) as a colorless solid. 1H-NMR (400 MHz, CDCl3) δ: 1.14 (9H, s), 2.09 (1H, brs), 3.44 (1H, t, J=5.0 Hz), 3.55-3.63 (2H, m), 3.70-3.73 (1H, m), 3.72 (3H, s), 3.91 (1H, d, J=13.2 Hz), 7.23-7.38 (5H, m). MS (ESI) m/z: 266 [(M+1)]+
References:
EP2380892,2011,A1 Location in patent:Page/Page column 74

100-52-7
952 suppliers
$20.37/250g

670278-82-7
32 suppliers
$60.00/100mg
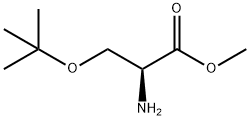
17083-26-0
33 suppliers
inquiry

670278-82-7
32 suppliers
$60.00/100mg