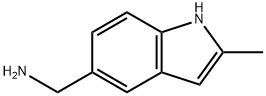
C-(2-METHYL-1H-INDOL-5-YL)-METHYLAMINE synthesis
- Product Name:C-(2-METHYL-1H-INDOL-5-YL)-METHYLAMINE
- CAS Number:36798-25-1
- Molecular formula:C10H12N2
- Molecular Weight:160.22
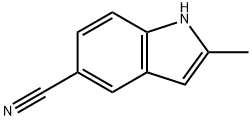
36798-24-0
28 suppliers
inquiry

36798-25-1
48 suppliers
$45.00/50mg
Yield:36798-25-1 97%
Reaction Conditions:
Stage #1: 2-methyl-1H-indole-5-carbonitrilewith lithium aluminium tetrahydride in tetrahydrofuran at 5 - 7; for 1.5 h;Reflux;
Stage #2: with water;sodium hydroxide in tetrahydrofuran; for 0.166667 h;Cooling with ice;
Steps:
1.2
LiAlH4 (9.70 g, 255 mmol) was suspended in THF (400 mL) and the resulting suspension was cooled in a ice/water bath. A solution of 2-methyl-1H-indole-5-carbonitrile (11.4 g, 73 mmol) in THF (100 mL) was added dropwise over 30 minutes while keeping the internal temperature at 5-7° C. The mixture was then heated to reflux for 1 h. The mixture was cooled in an ice-water bath. To this mixture was sequentially added 20 ml water, 10 ml sodium hydroxide-solution (5M) and 50 ml water and the mixture was stirred for 10 minutes. A generous amount of MgSO4 was added. The mixture was stirred for additional 10 minutes and then filtered. The residue was thoroughly extracted with THF. To the combined filtrates was added EtOAc (1 L) and this solution was dried with MgSO4. The mixture was filtered and evaporated to dryness to give the title compound as a light-yellow powder (11.9 g, 97%).1H NMR (500 MHz, DMSO) δ 10.76 (s, 1H), 7.31 (s, 1H), 7.18 (d, J=8.2 Hz, 1H), 6.95 (d, J=8.2 Hz, 1H), 6.05 (s, 1H), 3.73 (s, 2H), 2.36 (s, 3H).
References:
US2012/252853,2012,A1 Location in patent:Page/Page column 13-14
![2-[(2-methyl-1H-indol-5-yl)methyl]isoindole-1,3-dione](/CAS/20180531/GIF/58867-56-4.gif)
58867-56-4
2 suppliers
inquiry

36798-25-1
48 suppliers
$45.00/50mg
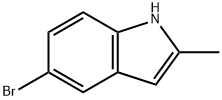
1075-34-9
148 suppliers
$8.00/100mg

36798-25-1
48 suppliers
$45.00/50mg
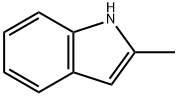
95-20-5
407 suppliers
$5.00/10g

36798-25-1
48 suppliers
$45.00/50mg
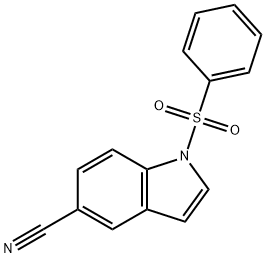
144340-21-6
11 suppliers
inquiry

36798-25-1
48 suppliers
$45.00/50mg