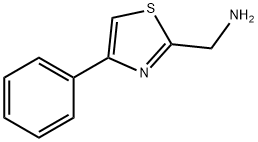
C-(4-PHENYL-THIAZOL-2-YL)-METHYLAMINE synthesis
- Product Name:C-(4-PHENYL-THIAZOL-2-YL)-METHYLAMINE
- CAS Number:90916-45-3
- Molecular formula:C10H10N2S
- Molecular Weight:190.26

381184-91-4
9 suppliers
$452.00/1g

90916-45-3
38 suppliers
$45.00/25mg
Yield: 77%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;water at 100; for 24 h;
Steps:
52 (4-Phenylthiazol-2-yl)methanamine
6N HCl (4.5 mL) was added to a solution of N-((4-phenylthiazol-2-yl)methyl)benzamide (150 mg, 0.51 mmol) in dioxane (10 mL) and the reaction mixture was stirred at 100 °C for 24 h (reaction monitored by TLC, eluant CHCl3/MeOH).
Reaction mixture was concentrated under reduced pressure and the residue was dissolved in water.
The aqueous layer was washed twice with EtOAc.
The pH of the aqueous layer was then adjusted to pH ∼9 using 10% NaHCO3 and the organic product was extracted with EtOAc.
The organic layer was washed with brine and concentrated under reduced pressure to yield (4-phenylthiazol-2-yl)methanamine (75 mg, yield 77%) as orange colored liquid, which was carried through without further purification. 1H NMR (300MHz, CDCl3) δ 7.91 - 7.88 (m, 2H), 7.45 - 7.40 (m, 3H), 7.36 - 7.31 (m, 1 H), 4.25 (br s, 2H), 3.79 - 3.75 (m, 1 H), 3.68 - 3.63 (m, 1 H). MS (ESI) m/z: Calculated for C10H10N2S: 190.06; found: 191.2 (M+H)+.
References:
Tempero Pharmaceuticals, Inc.;BALOGLU, Erkan;GHOSH, Shomir;LOBERA, Mercedes;SCHMIDT, Darby EP2533783, 2015, B1 Location in patent:Paragraph 0497-0498
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

90916-45-3
38 suppliers
$45.00/25mg
70-11-1
294 suppliers
$10.00/25g

90916-45-3
38 suppliers
$45.00/25mg